Entrainment of Arteriole Vasomotor Fluctuations by Neural Activity Is a Basis of Blood-Oxygenation-Level-Dependent "Resting-State" Connectivity
- PMID: 29107517
- PMCID: PMC5851777
- DOI: 10.1016/j.neuron.2017.10.012
Entrainment of Arteriole Vasomotor Fluctuations by Neural Activity Is a Basis of Blood-Oxygenation-Level-Dependent "Resting-State" Connectivity
Abstract
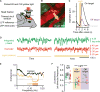
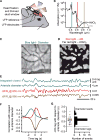
Resting-state signals in blood-oxygenation-level-dependent (BOLD) imaging are used to parcellate brain regions and define "functional connections" between regions. Yet a physiological link between fluctuations in blood oxygenation with those in neuronal signaling pathways is missing. We present evidence from studies on mouse cortex that modulation of vasomotion, i.e., intrinsic ultra-slow (0.1 Hz) fluctuations in arteriole diameter, provides this link. First, ultra-slow fluctuations in neuronal signaling, which occur as an envelope over γ-band activity, entrains vasomotion. Second, optogenetic manipulations confirm that entrainment is unidirectional. Third, co-fluctuations in the diameter of pairs of arterioles within the same hemisphere diminish to chance for separations >1.4 mm. Yet the diameters of arterioles in distant (>5 mm), mirrored transhemispheric sites strongly co-fluctuate; these correlations are diminished in acallosal mice. Fourth, fluctuations in arteriole diameter coherently drive fluctuations in blood oxygenation. Thus, entrainment of vasomotion links neuronal pathways to functional connections.
Keywords: Coupled oscillators; Functional magnetic resonant imaging; Hemodynamics; Intrinsic optical imaging; Optogenetics; Two-photon imaging.
Copyright © 2017. Published by Elsevier Inc.
Figures




Comment in
-
Good Vibrations: Resting-State Functional Connectivity Reflects Entrainment of Vasomotion.Neuron. 2017 Nov 15;96(4):716-717. doi: 10.1016/j.neuron.2017.10.035. Neuron. 2017. PMID: 29144969
References
-
- Ahrens KF, Kleinfeld D. Current flow in vibrissa motor cortex can phase-lock with exploratory rhythmic whisking in rat. J Neurophysiol. 2004;92:1700–1707. - PubMed
-
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. - PubMed
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

