A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss
- PMID: 29107524
- PMCID: PMC5699918
- DOI: 10.1016/j.molmet.2017.10.003
A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss
Abstract
Objective: Previous studies have reported that polyphenol-rich extracts from various sources can prevent obesity and associated gastro-hepatic and metabolic disorders in diet-induced obese (DIO) mice. However, whether such extracts can reverse obesity-linked metabolic alterations remains unknown. In the present study, we aimed to investigate the potential of a polyphenol-rich extract from cranberry (CE) to reverse obesity and associated metabolic disorders in DIO-mice.
Methods: Mice were pre-fed either a Chow or a High Fat-High Sucrose (HFHS) diet for 13 weeks to induce obesity and then treated either with CE (200 mg/kg, Chow + CE, HFHS + CE) or vehicle (Chow, HFHS) for 8 additional weeks.
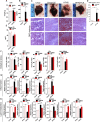
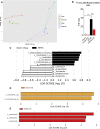
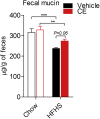
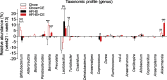
Results: CE did not reverse weight gain or fat mass accretion in Chow- or HFHS-fed mice. However, HFHS + CE fully reversed hepatic steatosis and this was linked to upregulation of genes involved in lipid catabolism (e.g., PPARα) and downregulation of several pro-inflammatory genes (eg, COX2, TNFα) in the liver. These findings were associated with improved glucose tolerance and normalization of insulin sensitivity in HFHS + CE mice. The gut microbiota of HFHS + CE mice was characterized by lower Firmicutes to Bacteroidetes ratio and a drastic expansion of Akkermansia muciniphila and, to a lesser extent, of Barnesiella spp, as compared to HFHS controls.
Conclusions: Taken together, our findings demonstrate that CE, without impacting body weight or adiposity, can fully reverse HFHS diet-induced insulin resistance and hepatic steatosis while triggering A. muciniphila blooming in the gut microbiota, thus underscoring the gut-liver axis as a primary target of cranberry polyphenols.
Keywords: Akkermansia; Barnesiella; Flavonoids; Obesity; Vaccinium macrocarpon.
Copyright © 2017 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures



References
-
- Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. - PMC - PubMed
-
- Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

