Caudate Microstimulation Increases Value of Specific Choices
- PMID: 29107551
- PMCID: PMC5773342
- DOI: 10.1016/j.cub.2017.09.051
Caudate Microstimulation Increases Value of Specific Choices
Abstract
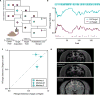
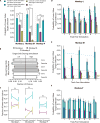
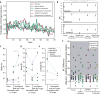
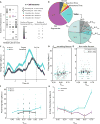
Value-based decision-making involves an assessment of the value of items available and the actions required to obtain them. The basal ganglia are highly implicated in action selection and goal-directed behavior [1-4], and the striatum in particular plays a critical role in arbitrating between competing choices [5-9]. Previous work has demonstrated that neural activity in the caudate nucleus is modulated by task-relevant action values [6, 8]. Nonetheless, how value is represented and maintained in the striatum remains unclear since decision-making in these tasks relied on spatially lateralized responses, confounding the ability to generalize to a more abstract choice task [6, 8, 9]. Here, we investigate striatal value representations by applying caudate electrical stimulation in macaque monkeys (n = 3) to bias decision-making in a task that divorces the value of a stimulus from motor action. Electrical microstimulation is known to induce neural plasticity [10, 11], and caudate microstimulation in primates has been shown to accelerate associative learning [12, 13]. Our results indicate that stimulation paired with a particular stimulus increases selection of that stimulus, and this effect was stimulus dependent and action independent. The modulation of choice behavior using microstimulation was best modeled as resulting from changes in stimulus value. Caudate neural recordings (n = 1) show that changes in value-coding neuron activity are stimulus value dependent. We argue that caudate microstimulation can differentially increase stimulus values independent of action, and unilateral manipulations of value are sufficient to mediate choice behavior. These results support potential future applications of microstimulation to correct maladaptive plasticity underlying dysfunctional decision-making related to neuropsychiatric conditions.
Keywords: caudate; decision-making; macaque monkey; microstimulation; reinforcement learning; striatum; value.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

