Klotho Improves Cardiac Function by Suppressing Reactive Oxygen Species (ROS) Mediated Apoptosis by Modulating Mapks/Nrf2 Signaling in Doxorubicin-Induced Cardiotoxicity
- PMID: 29107939
- PMCID: PMC5687120
- DOI: 10.12659/msm.907449
Klotho Improves Cardiac Function by Suppressing Reactive Oxygen Species (ROS) Mediated Apoptosis by Modulating Mapks/Nrf2 Signaling in Doxorubicin-Induced Cardiotoxicity
Abstract
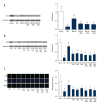
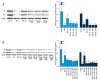
BACKGROUND Anthracyclines-induced cardiotoxicity has become one of the major restrictions of their clinical applications. Klotho showed cardioprotective effects. This study aimed to investigate the effects and possible mechanisms of klotho on doxorubicin (DOX)-induced cardiotoxicity. MATERIAL AND METHODS Rats and isolated myocytes were exposed to DOX and treated with exogenous klotho. Specific inhibitors and siRNAs silencing MAPKs were also used to treat the animals and/or myocytes. An invasive hemodynamic method was used to determine cardiac functions. Intracellular ROS generation was evaluated by DHE staining. Western blotting was used to determine the phosphorylation levels of JNK, ERK, and p38 MAPKs in plasma extracts and Nrf2 in nuclear extracts. Nuclear translocation of Nrf2 in myocytes was evaluated by immunohistochemistry. Cell apoptosis was evaluated by TUNEL assay and flow cytometry. RESULTS Klotho treatment improved DOX-induced cardiac dysfunction in rats. The DOX-induced ROS accumulation and cardiac apoptosis were attenuated by klotho. Impaired phosphorylations of MAPKs, Nrf2 translocation and expression levels of HO1 and Prx1 were also attenuated by klotho treatment. However, the anti-oxidant and anti-apoptotic effects of klotho on DOX-exposed myocardium and myocytes were impaired by both specific inhibitors and siRNAs against MAPKs. Moreover, the recovery effects of klotho on phosphorylations of MAPKs, Nrf2 translocation and expression levels of HO1 and Prx1 were also impaired by specific inhibitors and siRNAs against MAPKs. CONCLUSIONS By recovering the activation of MAPKs signaling, klotho improved cardiac function loss which was triggered by DOX-induced ROS mediated cardiac apoptosis.
Figures



References
-
- Lou Y, Wang Z, Xu Y, et al. Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int J Mol Med. 2015;36(3):873–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

