MeCP2 mediated dysfunction in senescent EPCs
- PMID: 29108229
- PMCID: PMC5667962
- DOI: 10.18632/oncotarget.20961
MeCP2 mediated dysfunction in senescent EPCs
Abstract
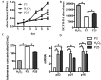
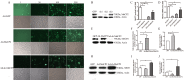
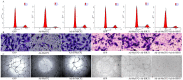
Aging endothelial progenitor cells (EPCs) exhibit functional impairment in terms of proliferation, migration and survival. SIRT1 plays an important role in improving EPCs function. MeCP2, another important epigenetic regulator, is involved in regulating many life-related activities such as cell growth, death and senescence. Here we aim to explore the effect of MeCP2 on the functional activities of senescent EPCs and the underlying mechanisms. By using western blot and real-time PCR, we found that the expression levels of MeCP2 were up-regulated and SIRT1 were down-regulated with replicative senescence and H2O2-induced senescence. Through transduction with adenoviral vectors, EPCs overexpressing MeCP2 had significantly reduced EPCs function, and silencing MeCP2 improved EPCs function. In addition, the protein and mRNA levels of SIRT1 were decreased with MeCP2 overexpression and increased with MeCP2 knockdown. Through co-transfection of EPCs with MeCP2 and SIRT1, we observed that SIRT1 could reverse the effects of MeCP2 on EPCs. In summary, our work demonstrated that MeCP2 inhibited SIRT1 in senescent EPCs.
Keywords: EPCs; Gerotarget; MeCP2; SIRT1; senescence.
Conflict of interest statement
CONFLICTS OF INTEREST There are no conflicts of interest to disclose.
Figures




References
-
- Altabas V, Altabas K, Kirigin L. Endothelial progenitor cells (EPCs) in ageing and age-related diseases: How currently available treatment modalities affect EPC biology, atherosclerosis, and cardiovascular outcomes. Mech Ageing Dev. 2016;159:49–62. - PubMed
-
- Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. - PubMed
-
- Simper D, Wang S, Deb A, Holmes D, McGregor C, Frantz R, Kushwaha SS, Caplice NM. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation. 2003;108:143–149. - PubMed
-
- Lee SH, Lee JH, Yoo SY, Hur J, Kim HS, Kwon SM. Hypoxia inhibits cellular senescence to restore the therapeutic potential of old human endothelial progenitor cells via the hypoxia-inducible factor-1alpha-TWIST-p21 axis. Arterioscler Thromb Vasc Biol. 2013;33:2407–2414. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

