Baseline factors associated with response to ruxolitinib: an independent study on 408 patients with myelofibrosis
- PMID: 29108288
- PMCID: PMC5668021
- DOI: 10.18632/oncotarget.18674
Baseline factors associated with response to ruxolitinib: an independent study on 408 patients with myelofibrosis
Abstract
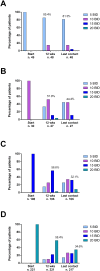
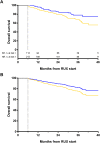
In patients with Myelofibrosis (MF) treated with ruxolitinib (RUX), the response is unpredictable at therapy start. We retrospectively evaluated the impact of clinical/laboratory factors on responses in 408 patients treated with RUX according to prescribing obligations in 18 Italian Hematology Centers. At 6 months, 114 out of 327 (34.9%) evaluable patients achieved a spleen response. By multivariable Cox proportional hazard regression model, pre-treatment factors negatively correlating with spleen response were: high/intermediate-2 IPSS risk (p=0.024), large splenomegaly (p=0.017), transfusion dependency (p=0.022), platelet count <200×109/l (p=0.028), and a time-interval between MF diagnosis and RUX start >2 years (p=0.048). Also, patients treated with higher (≥10 mg BID) average RUX doses in the first 12 weeks achieved higher response rates (p=0.019). After adjustment for IPSS risk, patients in spleen response at 6 months showed only a trend for better survival compared to non-responders. At 6 months, symptoms response was achieved by 85.5% of 344 evaluable patients; only a higher (>20) Total Symptom Score significantly correlated with lower probability of response (p<0.001). Increased disease severity, a delay in RUX start and titrated doses <10 mg BID were associated with patients achievinglower response rates. An early treatment and higher RUX doses may achieve better therapeutic results.
Keywords: myelofibrosis; predictive factors; response; ruxolitinib; splenomegaly.
Conflict of interest statement
CONFLICTS OF INTEREST MT has acted as consultant and received honoraria from Novartis, BMS and ARIAD. MBo declares research funding from Novartis. GAP, FDR and AC report personal fees and non-financial support from NOVARTIS, from null, outside the submitted work.
Figures



References
-
- Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1262–1271. - PubMed
-
- Reilly JT, McMullin MF, Beer PA, Butt N, Conneally E, Duncombe A, Green AR, Michaeel NG, Gilleece MH, Hall GW, Knapper S, Mead A, Mesa RA, et al. Guideline for the diagnosis and management of myelofibrosis. Br J Haematol. 2012;158:453–471. - PubMed
-
- Geyer HL, Kosiorek H, Dueck AC, Scherber R, Slot S, Zweegman S, Te Boekhorst PA, Senyak Z, Schouten HC, Sackmann F, Fuentes AK, Hernández-Maraver D, Pahl HL, et al. Associations between gender, disease features and symptom burden in patients with myeloproliferative neoplasms: an analysis by the MPN QOL International Working Group. Haematologica. 2017;102:85–93. - PMC - PubMed
-
- Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, Vannucchi AM, Mesa RA, Demory JL, Barosi G, Rumi E, Tefferi A. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

