Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models
- PMID: 29108957
- PMCID: PMC5801150
- DOI: 10.1016/j.bbagen.2017.11.005
Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models
Abstract
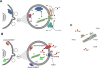
Background: Matrix vesicles (MVs) are released from hypertrophic chondrocytes and from mature osteoblasts, the cells responsible for endochondral and membranous ossification. Under pathological conditions, they can also be released from cells of non-skeletal tissues such as vascular smooth muscle cells. MVs are extracellular vesicles of approximately 100-300nm diameter harboring the biochemical machinery needed to induce mineralization.
Scope of the review: The review comprehensively delineates our current knowledge of MV biology and highlights open questions aiming to stimulate further research. The review is constructed as a series of questions addressing issues of MVs ranging from their biogenesis and functions, to biomimetic models. It critically evaluates experimental data including their isolation and characterization methods, like lipidomics, proteomics, transmission electron microscopy, atomic force microscopy and proteoliposome models mimicking MVs.
Major conclusions: MVs have a relatively well-defined function as initiators of mineralization. They bind to collagen and their composition reflects the composition of lipid rafts. We call attention to the as yet unclear mechanisms leading to the biogenesis of MVs, and how minerals form and when they are formed. We discuss the prospects of employing upcoming experimental models to deepen our understanding of MV-mediated mineralization and mineralization disorders such as the use of reconstituted lipid vesicles, proteoliposomes and, native sample preparations and high-resolution technologies.
General significance: MVs have been extensively investigated owing to their roles in skeletal and ectopic mineralization. MVs serve as a model system for lipid raft structures, and for the mechanisms of genesis and release of extracellular vesicles.
Keywords: Atomic force microscopy; Electron microscopy; Lipid raft; Matrix vesicles; Mineralization; Proteoliposomes.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures





References
-
- Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. - PubMed
-
- Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest. 2010;90:1549–1557. - PubMed
-
- Stratmann U, Schaarschmidt K, Wiesmann HP, Plate U, Höhling HJ. Mineralization during matrix-vesicle-mediated mantle dentine formation in molars of albino rats: a microanalytical and ultrastructural study. Cell Tissue Res. 1996;284:223–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

