Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer
- PMID: 29109077
- PMCID: PMC5712264
- DOI: 10.1158/2326-6066.CIR-17-0189
Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer
Abstract
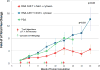
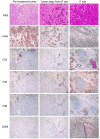
Chimeric antigen receptors (CAR) are synthetic molecules that provide new specificities to T cells. Although successful in treatment of hematologic malignancies, CAR T cells are ineffective for solid tumors to date. We found that the cell-surface molecule c-Met was expressed in ∼50% of breast tumors, prompting the construction of a CAR T cell specific for c-Met, which halted tumor growth in immune-incompetent mice with tumor xenografts. We then evaluated the safety and feasibility of treating metastatic breast cancer with intratumoral administration of mRNA-transfected c-Met-CAR T cells in a phase 0 clinical trial (NCT01837602). Introducing the CAR construct via mRNA ensured safety by limiting the nontumor cell effects (on-target/off-tumor) of targeting c-Met. Patients with metastatic breast cancer with accessible cutaneous or lymph node metastases received a single intratumoral injection of 3 × 107 or 3 × 108 cells. CAR T mRNA was detectable in peripheral blood and in the injected tumor tissues after intratumoral injection in 2 and 4 patients, respectively. mRNA c-Met-CAR T cell injections were well tolerated, as none of the patients had study drug-related adverse effects greater than grade 1. Tumors treated with intratumoral injected mRNA c-Met-CAR T cells were excised and analyzed by immunohistochemistry, revealing extensive tumor necrosis at the injection site, cellular debris, loss of c-Met immunoreactivity, all surrounded by macrophages at the leading edges and within necrotic zones. We conclude that intratumoral injections of mRNA c-Met-CAR T cells are well tolerated and evoke an inflammatory response within tumors. Cancer Immunol Res; 5(12); 1152-61. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
The University of Pennsylvania has a strategic alliance with Novartis for the development of chimeric antigen receptors. This arrangement is managed in accordance with the University of Pennsylvania’s Conflict of Interest Policy. The authors are in compliance with this policy. Y.Z., B.L.L, and C.H.J. are co-founders of Tmunity Therapeutics.
Figures

References
-
- Porter DL, Hwang W-T, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. - DOI - PMC - PubMed
-
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

