Peptide splicing by the proteasome
- PMID: 29109146
- PMCID: PMC5743089
- DOI: 10.1074/jbc.R117.807560
Peptide splicing by the proteasome
Abstract
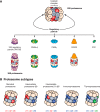
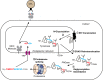
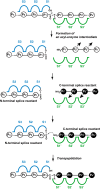
The proteasome is the major protease responsible for the production of antigenic peptides recognized by CD8+ cytolytic T cells (CTL). These peptides, generally 8-10 amino acids long, are presented at the cell surface by major histocompatibility complex (MHC) class I molecules. Originally, these peptides were believed to be solely derived from linear fragments of proteins, but this concept was challenged several years ago by the isolation of anti-tumor CTL that recognized spliced peptides, i.e. peptides composed of fragments distant in the parental protein. The splicing process was shown to occur in the proteasome through a transpeptidation reaction involving an acyl-enzyme intermediate. Here, we review the steps that led to the discovery of spliced peptides as well as the recent advances that uncover the unexpected importance of spliced peptides in the composition of the MHC class I repertoire.
Keywords: MHC class I; antigen presentation; antigen processing; cytolytic T lymphocytes; major histocompatibility complex (MHC); peptides; proteasome; spliced peptides.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Rock K. L., Gramm C., Rothstein L., Clark K., Stein R., Dick L., Hwang D., and Goldberg A. L. (1994) Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78, 761–771 - PubMed
-
- Androlewicz M. J., Anderson K. S., and Cresswell P. (1993) Evidence that transporters associated with antigen processing translocate a major histocompatibility complex class I-binding peptide into the endoplasmic reticulum in an ATP-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 90, 9130–9134 - PMC - PubMed
-
- Saric T., Chang S. C., Hattori A., York I. A., Markant S., Rock K. L., Tsujimoto M., and Goldberg A. L. (2002) An IFN-γ-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat. Immunol. 3, 1169–1176 - PubMed
-
- Saveanu L., Carroll O., Lindo V., Del Val M., Lopez D., Lepelletier Y., Greer F., Schomburg L., Fruci D., Niedermann G., and van Endert P. M. (2005) Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat. Immunol. 6, 689–697 - PubMed
-
- York I. A., Chang S. C., Saric T., Keys J. A., Favreau J. M., Goldberg A. L., and Rock K. L. (2002) The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat. Immunol. 3, 1177–1184 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

