HDAC8 substrate selectivity is determined by long- and short-range interactions leading to enhanced reactivity for full-length histone substrates compared with peptides
- PMID: 29109148
- PMCID: PMC5766737
- DOI: 10.1074/jbc.M117.811026
HDAC8 substrate selectivity is determined by long- and short-range interactions leading to enhanced reactivity for full-length histone substrates compared with peptides
Abstract
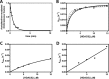
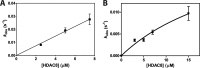
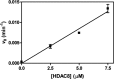
Histone deacetylases (HDACs) catalyze deacetylation of acetyl-lysine residues within proteins. To date, HDAC substrate specificity and selectivity have been largely estimated using peptide substrates. However, it is unclear whether peptide substrates accurately reflect the substrate selectivity of HDAC8 toward full-length proteins. Here, we compare HDAC8 substrate selectivity in the context of peptides, full-length proteins, and protein-nucleic acid complexes. We demonstrate that HDAC8 catalyzes deacetylation of tetrameric histone (H3/H4) substrates with catalytic efficiencies that are 40-300-fold higher than those for corresponding peptide substrates. Thus, we conclude that additional contacts with protein substrates enhance catalytic efficiency. However, the catalytic efficiency decreases for larger multiprotein complexes. These differences in HDAC8 substrate selectivity for peptides and full-length proteins suggest that HDAC8 substrate preference is based on a combination of short- and long-range interactions. In summary, this work presents detailed kinetics for HDAC8-catalyzed deacetylation of singly-acetylated, full-length protein substrates, revealing that HDAC8 substrate selectivity is determined by multiple factors. These insights provide a foundation for understanding recognition of full-length proteins by HDACs.
Keywords: enzyme kinetics; enzyme turnover; histone; histone acetylation; histone deacetylase (HDAC); non-natural amino acid incorporation; nucleosome; peptides; protein complex.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Khochbin S., Verdel A., Lemercier C., and Seigneurin-Berny D. (2001) Functional significance of histone deacetylase diversity. Curr. Opin. Genet. Dev. 11, 162–166 - PubMed
-
- Glozak M. A., Sengupta N., Zhang X., and Seto E. (2005) Acetylation and deacetylation of non-histone proteins. Gene 363, 15–23 - PubMed
-
- Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., and Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

