Combating Multidrug-Resistant Pathogens with Host-Directed Nonantibiotic Therapeutics
- PMID: 29109161
- PMCID: PMC5740341
- DOI: 10.1128/AAC.01943-17
Combating Multidrug-Resistant Pathogens with Host-Directed Nonantibiotic Therapeutics
Abstract
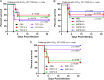
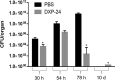
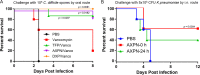
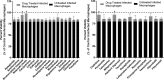
Earlier, we reported that three Food and Drug Administration-approved drugs, trifluoperazine (TFP; an antipsychotic), amoxapine (AXPN; an antidepressant), and doxapram (DXP; a breathing stimulant), identified from an in vitro murine macrophage cytotoxicity screen, provided mice with 40 to 60% protection against pneumonic plague when administered at the time of infection for 1 to 3 days. In the present study, the therapeutic potential of these drugs against pneumonic plague in mice was further evaluated when they were administered at up to 48 h postinfection. While the efficacy of TFP was somewhat diminished as treatment was delayed to 24 h, the protection of mice with AXPN and DXP increased as treatment was progressively delayed to 24 h. At 48 h postinfection, these drugs provided the animals with significant protection (up to 100%) against challenge with the agent of pneumonic or bubonic plague when they were administered in combination with levofloxacin. Likewise, when they were used in combination with vancomycin, all three drugs provided mice with 80 to 100% protection from fatal oral Clostridium difficile infection when they were administered at 24 h postinfection. Furthermore, AXPN provided 40 to 60% protection against respiratory infection with Klebsiella pneumoniae when it was administered at the time of infection or at 24 h postinfection. Using the same in vitro cytotoxicity assay, we identified an additional 76/780 nonantibiotic drugs effective against K. pneumoniae For Acinetobacter baumannii, 121 nonantibiotic drugs were identified to inhibit bacterium-induced cytotoxicity in murine macrophages. Of these 121 drugs, 13 inhibited the macrophage cytotoxicity induced by two additional multiple-antibiotic-resistant strains. Six of these drugs decreased the intracellular survival of all three A. baumannii strains in macrophages. These results provided further evidence of the broad applicability and utilization of drug repurposing screening to identify new therapeutics to combat multidrug-resistant pathogens of public health concern.
Keywords: Acinetobacter baumannii; Clostridium difficile; Klebsiella pneumoniae; Yersinia pestis; bubonic plague; in vitro assays; mouse models; new therapeutics; pneumonic plague.
Copyright © 2017 American Society for Microbiology.
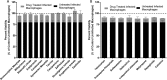
Figures



References
-
- CDC. 2013. Antibiotic resistance threats in the United States, 2013. Antibiotic/antimicrobial resistance. CDC, Atlanta, GA.
-
- O'Neill J. 2014. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. In Review on antimicrobial resistance. HM Government and The Wellcome Trust, London, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

