Development of Subunit Vaccines That Provide High-Level Protection and Sterilizing Immunity against Acute Inhalational Melioidosis
- PMID: 29109172
- PMCID: PMC5736816
- DOI: 10.1128/IAI.00724-17
Development of Subunit Vaccines That Provide High-Level Protection and Sterilizing Immunity against Acute Inhalational Melioidosis
Abstract
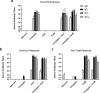
Burkholderia pseudomallei, the etiologic agent of melioidosis, causes severe disease in humans and animals. Diagnosis and treatment of melioidosis can be challenging, and no licensed vaccines currently exist. Several studies have shown that this pathogen expresses a variety of structurally conserved protective antigens that include cell surface polysaccharides and cell-associated and cell-secreted proteins. Based on those findings, such antigens have become important components of the subunit vaccine candidates that we are currently developing. In the present study, the 6-deoxyheptan capsular polysaccharide (CPS) from B. pseudomallei was purified, chemically activated, and covalently linked to recombinant CRM197 diphtheria toxin mutant (CRM197) to produce CPS-CRM197. Additionally, tandem nickel-cobalt affinity chromatography was used to prepare highly purified recombinant B. pseudomallei Hcp1 and TssM proteins. Immunization of C57BL/6 mice with CPS-CRM197 produced high-titer IgG and opsonizing antibody responses against the CPS component of the glycoconjugate, while immunization with Hcp1 and TssM produced high-titer IgG and robust gamma interferon-secreting T cell responses against the proteins. Extending upon these studies, we found that when mice were vaccinated with a combination of CPS-CRM197 and Hcp1, 100% of the mice survived a lethal inhalational challenge with B. pseudomallei Remarkably, 70% of the survivors had no culturable bacteria in their lungs, livers, or spleens, indicating that the vaccine formulation had generated sterilizing immune responses. Collectively, these studies help to better establish surrogates of antigen-induced immunity against B. pseudomallei as well as provide valuable insights toward the development of a safe, affordable, and effective melioidosis vaccine.
Keywords: Burkholderia pseudomallei; Hcp1; capsule; glycoconjugate; immunity; inhalation; melioidosis; mouse; protection; vaccines.
Copyright © 2017 Burtnick et al.
Figures



Similar articles
-
Development of Melioidosis Subunit Vaccines Using an Enzymatically Inactive Burkholderia pseudomallei AhpC.Infect Immun. 2022 Aug 18;90(8):e0022222. doi: 10.1128/iai.00222-22. Epub 2022 Jul 11. Infect Immun. 2022. PMID: 35862715 Free PMC article.
-
Multicomponent Gold-Linked Glycoconjugate Vaccine Elicits Antigen-Specific Humoral and Mixed TH1-TH17 Immunity, Correlated with Increased Protection against Burkholderia pseudomallei.mBio. 2021 Jun 29;12(3):e0122721. doi: 10.1128/mBio.01227-21. Epub 2021 Jun 29. mBio. 2021. PMID: 34182777 Free PMC article.
-
Development of capsular polysaccharide-based glycoconjugates for immunization against melioidosis and glanders.Front Cell Infect Microbiol. 2012 Aug 15;2:108. doi: 10.3389/fcimb.2012.00108. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22912938 Free PMC article.
-
Development of Burkholderia mallei and pseudomallei vaccines.Front Cell Infect Microbiol. 2013 Mar 11;3:10. doi: 10.3389/fcimb.2013.00010. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 23508691 Free PMC article. Review.
-
Novel multi-component vaccine approaches for Burkholderia pseudomallei.Clin Exp Immunol. 2019 May;196(2):178-188. doi: 10.1111/cei.13286. Epub 2019 Apr 8. Clin Exp Immunol. 2019. PMID: 30963550 Free PMC article. Review.
Cited by
-
Combating the great mimicker: latest progress in the development of Burkholderia pseudomallei vaccines.Expert Rev Vaccines. 2020 Jul;19(7):653-660. doi: 10.1080/14760584.2020.1791089. Epub 2020 Jul 15. Expert Rev Vaccines. 2020. PMID: 32669008 Free PMC article. Review.
-
Human Melioidosis.Clin Microbiol Rev. 2020 Mar 11;33(2):e00006-19. doi: 10.1128/CMR.00006-19. Print 2020 Mar 18. Clin Microbiol Rev. 2020. PMID: 32161067 Free PMC article. Review.
-
Functional Activities of O-Polysaccharide and Hemolysin Coregulated Protein 1 Specific Antibodies Isolated from Melioidosis Patients.Infect Immun. 2022 Nov 17;90(11):e0021422. doi: 10.1128/iai.00214-22. Epub 2022 Oct 13. Infect Immun. 2022. PMID: 36226942 Free PMC article.
-
Molecular epidemiology of Burkholderia pseudomallei in Hainan Province of China based on O-antigen.Infect Med (Beijing). 2024 Nov 8;3(4):100150. doi: 10.1016/j.imj.2024.100150. eCollection 2024 Dec. Infect Med (Beijing). 2024. PMID: 39697185 Free PMC article.
-
Layering vaccination with antibiotic therapy results in protection and clearance of Burkholderia pseudomallei in Balb/c mice.Infect Immun. 2024 Mar 12;92(3):e0045523. doi: 10.1128/iai.00455-23. Epub 2024 Jan 30. Infect Immun. 2024. PMID: 38289122 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

