Candidalysin Drives Epithelial Signaling, Neutrophil Recruitment, and Immunopathology at the Vaginal Mucosa
- PMID: 29109176
- PMCID: PMC5778364
- DOI: 10.1128/IAI.00645-17
Candidalysin Drives Epithelial Signaling, Neutrophil Recruitment, and Immunopathology at the Vaginal Mucosa
Abstract
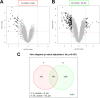
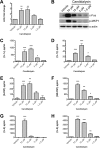
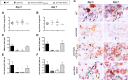
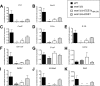
Unlike other forms of candidiasis, vulvovaginal candidiasis, caused primarily by the fungal pathogen Candida albicans, is a disease of immunocompetent and otherwise healthy women. Despite its prevalence, the fungal factors responsible for initiating symptomatic infection remain poorly understood. One of the hallmarks of vaginal candidiasis is the robust recruitment of neutrophils to the site of infection, which seemingly do not clear the fungus, but rather exacerbate disease symptomatology. Candidalysin, a newly discovered peptide toxin secreted by C. albicans hyphae during invasion, drives epithelial damage, immune activation, and phagocyte attraction. Therefore, we hypothesized that Candidalysin is crucial for vulvovaginal candidiasis immunopathology. Anti-Candida immune responses are anatomical-site specific, as effective gastrointestinal, oral, and vaginal immunities are uniquely compartmentalized. Thus, we aimed to identify the immunopathologic role of Candidalysin and downstream signaling events at the vaginal mucosa. Microarray analysis of C. albicans-infected human vaginal epithelium in vitro revealed signaling pathways involved in epithelial damage responses, barrier repair, and leukocyte activation. Moreover, treatment of A431 vaginal epithelial cells with Candidalysin induced dose-dependent proinflammatory cytokine responses (including interleukin 1α [IL-1α], IL-1β, and IL-8), damage, and activation of c-Fos and mitogen-activated protein kinase (MAPK) signaling, consistent with fungal challenge. Mice intravaginally challenged with C. albicans strains deficient in Candidalysin exhibited no differences in colonization compared to isogenic controls. However, significant decreases in neutrophil recruitment, damage, and proinflammatory cytokine expression were observed with these strains. Our findings demonstrate that Candidalysin is a key hypha-associated virulence determinant responsible for the immunopathogenesis of C. albicans vaginitis.
Keywords: Candida; Candidalysin; epithelial cells; immunopathogenesis; mucosal immunity; mucosal pathogens; mycology; vaginitis; vulvovaginal.
Copyright © 2018 American Society for Microbiology.
Figures


References
-
- Fidel PL Jr, Barousse M, Espinosa T, Ficarra M, Sturtevant J, Martin DH, Quayle AJ, Dunlap K. 2004. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun 72:2939–2946. doi:10.1128/IAI.72.5.2939-2946.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

