Graded Elevation of c-Jun in Schwann Cells In Vivo: Gene Dosage Determines Effects on Development, Remyelination, Tumorigenesis, and Hypomyelination
- PMID: 29109239
- PMCID: PMC5729195
- DOI: 10.1523/JNEUROSCI.0986-17.2017
Graded Elevation of c-Jun in Schwann Cells In Vivo: Gene Dosage Determines Effects on Development, Remyelination, Tumorigenesis, and Hypomyelination
Abstract
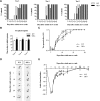
Schwann cell c-Jun is implicated in adaptive and maladaptive functions in peripheral nerves. In injured nerves, this transcription factor promotes the repair Schwann cell phenotype and regeneration and promotes Schwann-cell-mediated neurotrophic support in models of peripheral neuropathies. However, c-Jun is associated with tumor formation in some systems, potentially suppresses myelin genes, and has been implicated in demyelinating neuropathies. To clarify these issues and to determine how c-Jun levels determine its function, we have generated c-Jun OE/+ and c-Jun OE/OE mice with graded expression of c-Jun in Schwann cells and examined these lines during development, in adulthood, and after injury using RNA sequencing analysis, quantitative electron microscopic morphometry, Western blotting, and functional tests. Schwann cells are remarkably tolerant of elevated c-Jun because the nerves of c-Jun OE/+ mice, in which c-Jun is elevated ∼6-fold, are normal with the exception of modestly reduced myelin thickness. The stronger elevation of c-Jun in c-Jun OE/OE mice is, however, sufficient to induce significant hypomyelination pathology, implicating c-Jun as a potential player in demyelinating neuropathies. The tumor suppressor P19ARF is strongly activated in the nerves of these mice and, even in aged c-Jun OE/OE mice, there is no evidence of tumors. This is consistent with the fact that tumors do not form in injured nerves, although they contain proliferating Schwann cells with strikingly elevated c-Jun. Furthermore, in crushed nerves of c-Jun OE/+ mice, where c-Jun levels are overexpressed sufficiently to accelerate axonal regeneration, myelination and function are restored after injury.SIGNIFICANCE STATEMENT In injured and diseased nerves, the transcription factor c-Jun in Schwann cells is elevated and variously implicated in controlling beneficial or adverse functions, including trophic Schwann cell support for neurons, promotion of regeneration, tumorigenesis, and suppression of myelination. To analyze the functions of c-Jun, we have used transgenic mice with graded elevation of Schwann cell c-Jun. We show that high c-Jun elevation is a potential pathogenic mechanism because it inhibits myelination. Conversely, we did not find a link between c-Jun elevation and tumorigenesis. Modest c-Jun elevation, which is beneficial for regeneration, is well tolerated during Schwann cell development and in the adult and is compatible with restoration of myelination and nerve function after injury.
Keywords: PNS; Schwann; c-Jun; myelin; regeneration; tumorigenesis.
Copyright © 2017 Fazal, Gomez-Sanchez et al.
Figures








Comment in
-
c-Jun in Schwann Cells: Stay Away from Extremes.J Neurosci. 2018 Apr 4;38(14):3388-3390. doi: 10.1523/JNEUROSCI.0028-18.2018. J Neurosci. 2018. PMID: 29618544 Free PMC article. No abstract available.
References
-
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633–647. 10.1016/j.neuron.2012.06.021 - DOI - PMC - PubMed
-
- Arthur-Farraj PJ, Morgan CC, Adamowicz M, Gomez-Sanchez JA, Fazal SV, Beucher A, Razzaghi B, Rhona Mirsky R, Jessen KR, Aitman TJ (2017) Changes in the coding and non-coding transcriptome and DNA methylome that define the Schwann cell repair phenotype after nerve injury. Cell Rep 20:2719–2734. 10.1016/j.celrep.2017.08.064 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
