Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology
- PMID: 29109247
- PMCID: PMC5703340
- DOI: 10.1073/pnas.1717695114
Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology
Abstract
Implantable electrical probes have led to advances in neuroscience, brain-machine interfaces, and treatment of neurological diseases, yet they remain limited in several key aspects. Ideally, an electrical probe should be capable of recording from large numbers of neurons across multiple local circuits and, importantly, allow stable tracking of the evolution of these neurons over the entire course of study. Silicon probes based on microfabrication can yield large-scale, high-density recording but face challenges of chronic gliosis and instability due to mechanical and structural mismatch with the brain. Ultraflexible mesh electronics, on the other hand, have demonstrated negligible chronic immune response and stable long-term brain monitoring at single-neuron level, although, to date, it has been limited to 16 channels. Here, we present a scalable scheme for highly multiplexed mesh electronics probes to bridge the gap between scalability and flexibility, where 32 to 128 channels per probe were implemented while the crucial brain-like structure and mechanics were maintained. Combining this mesh design with multisite injection, we demonstrate stable 128-channel local field potential and single-unit recordings from multiple brain regions in awake restrained mice over 4 mo. In addition, the newly integrated mesh is used to validate stable chronic recordings in freely behaving mice. This scalable scheme for mesh electronics together with demonstrated long-term stability represent important progress toward the realization of ideal implantable electrical probes allowing for mapping and tracking single-neuron level circuit changes associated with learning, aging, and neurodegenerative diseases.
Keywords: large-scale neural recording; nano−bio interface; neural probe; tissue-like; ultraflexible.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


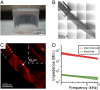


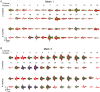

References
-
- Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–451. - PubMed
-
- Stanley GB. Reading and writing the neural code. Nat Neurosci. 2013;16:259–263. - PubMed
-
- Bargmann CI, Newsome WT. The Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiative and neurology. JAMA Neurol. 2014;71:675–676. - PubMed
-
- Borchers S, Himmelbach M, Logothetis N, Karnath HO. Direct electrical stimulation of human cortex—The gold standard for mapping brain functions? Nat Rev Neurosci. 2011;13:63–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

