Definition of the hypothalamic GnRH pulse generator in mice
- PMID: 29109258
- PMCID: PMC5703322
- DOI: 10.1073/pnas.1713897114
Definition of the hypothalamic GnRH pulse generator in mice
Abstract
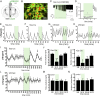
The pulsatile release of luteinizing hormone (LH) is critical for mammalian fertility. However, despite several decades of investigation, the identity of the neuronal network generating pulsatile reproductive hormone secretion remains unproven. We use here a variety of optogenetic approaches in freely behaving mice to evaluate the role of the arcuate nucleus kisspeptin (ARNKISS) neurons in LH pulse generation. Using GCaMP6 fiber photometry, we find that the ARNKISS neuron population exhibits brief (∼1 min) synchronized episodes of calcium activity occurring as frequently as every 9 min in gonadectomized mice. These ARNKISS population events were found to be near-perfectly correlated with pulsatile LH secretion. The selective optogenetic activation of ARNKISS neurons for 1 min generated pulses of LH in freely behaving mice, whereas inhibition with archaerhodopsin for 30 min suppressed LH pulsatility. Experiments aimed at resetting the activity of the ARNKISS neuron population with halorhodopsin were found to reset ongoing LH pulsatility. These observations indicate the ARNKISS neurons as the long-elusive hypothalamic pulse generator driving fertility.
Keywords: GnRH; arcuate nucleus; fertility; kisspeptin; pulse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Le Tissier P, et al. An updated view of hypothalamic-vascular-pituitary unit function and plasticity. Nat Rev Endocrinol. 2017;13:257–267. - PubMed
-
- Lightman SL, Conway-Campbell BL. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci. 2010;11:710–718. - PubMed
-
- Leng G, Brown D. The origins and significance of pulsatility in hormone secretion from the pituitary. J Neuroendocrinol. 1997;9:493–513. - PubMed
-
- Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87:850–853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

