Engineering of a membrane-triggered activity switch in coagulation factor VIIa
- PMID: 29109275
- PMCID: PMC5703266
- DOI: 10.1073/pnas.1618713114
Engineering of a membrane-triggered activity switch in coagulation factor VIIa
Abstract
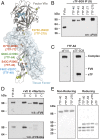
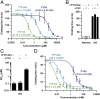
Recombinant factor VIIa (FVIIa) variants with increased activity offer the promise to improve the treatment of bleeding episodes in patients with inhibitor-complicated hemophilia. Here, an approach was adopted to enhance the activity of FVIIa by selectively optimizing substrate turnover at the membrane surface. Under physiological conditions, endogenous FVIIa engages its cell-localized cofactor tissue factor (TF), which stimulates activity through membrane-dependent substrate recognition and allosteric effects. To exploit these properties of TF, a covalent complex between FVIIa and the soluble ectodomain of TF (sTF) was engineered by introduction of a nonperturbing cystine bridge (FVIIa Q64C-sTF G109C) in the interface. Upon coexpression, FVIIa Q64C and sTF G109C spontaneously assembled into a covalent complex with functional properties similar to the noncovalent wild-type complex. Additional introduction of a FVIIa-M306D mutation to uncouple the sTF-mediated allosteric stimulation of FVIIa provided a final complex with FVIIa-like activity in solution, while exhibiting a two to three orders-of-magnitude increase in activity relative to FVIIa upon exposure to a procoagulant membrane. In a mouse model of hemophilia A, the complex normalized hemostasis upon vascular injury at a dose of 0.3 nmol/kg compared with 300 nmol/kg for FVIIa.
Keywords: coagulation; disulfide engineering; factor VIIa; serine proteases; tissue factor.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: A.L.N., A.B.S., H.L.H., P.S.G., J.B., K.L., M.K.-H., C.D.L., B.B.S., O.H.O., and H.Ø. are employed by Novo Nordisk A/S.
Figures


Comment in
-
The next best thing in factor VIIa.J Thromb Haemost. 2018 Oct;16(10):1911-1913. doi: 10.1111/jth.14250. Epub 2018 Aug 16. J Thromb Haemost. 2018. PMID: 30117258 No abstract available.
Similar articles
-
Beating tissue factor at its own game: Design and properties of a soluble tissue factor-independent coagulation factor VIIa.J Biol Chem. 2020 Jan 10;295(2):517-528. doi: 10.1074/jbc.RA119.009183. Epub 2019 Dec 4. J Biol Chem. 2020. PMID: 31801825 Free PMC article.
-
Evolutionary conservation of the allosteric activation of factor VIIa by tissue factor in lamprey.J Thromb Haemost. 2018 Apr;16(4):734-748. doi: 10.1111/jth.13968. Epub 2018 Mar 12. J Thromb Haemost. 2018. PMID: 29418058 Free PMC article.
-
Molecular Basis of Enhanced Activity in Factor VIIa-Trypsin Variants Conveys Insights into Tissue Factor-mediated Allosteric Regulation of Factor VIIa Activity.J Biol Chem. 2016 Feb 26;291(9):4671-83. doi: 10.1074/jbc.M115.698613. Epub 2015 Dec 22. J Biol Chem. 2016. PMID: 26694616 Free PMC article.
-
Cofactor-induced and mutational activity enhancement of coagulation factor VIIa.Cell Mol Life Sci. 2008 Mar;65(6):953-63. doi: 10.1007/s00018-007-7480-5. Cell Mol Life Sci. 2008. PMID: 18066494 Free PMC article. Review.
-
The action of high-dose factor VIIa (FVIIa) in a cell-based model of hemostasis.Dis Mon. 2003 Jan;49(1):14-21. doi: 10.1053/mda.2003.29504b. Dis Mon. 2003. PMID: 12525825 Review.
Cited by
-
Conformational Plasticity-Rigidity Axis of the Coagulation Factor VII Zymogen Elucidated by Atomistic Simulations of the N-Terminally Truncated Factor VIIa Protease Domain.Biomolecules. 2021 Apr 8;11(4):549. doi: 10.3390/biom11040549. Biomolecules. 2021. PMID: 33917935 Free PMC article.
-
Beating tissue factor at its own game: Design and properties of a soluble tissue factor-independent coagulation factor VIIa.J Biol Chem. 2020 Jan 10;295(2):517-528. doi: 10.1074/jbc.RA119.009183. Epub 2019 Dec 4. J Biol Chem. 2020. PMID: 31801825 Free PMC article.
-
Uncovering Membrane-Bound Models of Coagulation Factors by Combined Experimental and Computational Approaches.Thromb Haemost. 2021 Sep;121(9):1122-1137. doi: 10.1055/s-0040-1722187. Epub 2021 Jul 2. Thromb Haemost. 2021. PMID: 34214998 Free PMC article. Review.
References
-
- Mann KG, Butenas S, Brummel K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol. 2003;23:17–25. - PubMed
-
- Banner DW, et al. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380:41–46. - PubMed
-
- McCallum CD, Hapak RC, Neuenschwander PF, Morrissey JH, Johnson AE. The location of the active site of blood coagulation factor VIIa above the membrane surface and its reorientation upon association with tissue factor. A fluorescence energy transfer study. J Biol Chem. 1996;271:28168–28175. - PubMed
-
- Baugh RJ, Dickinson CD, Ruf W, Krishnaswamy S. Exosite interactions determine the affinity of factor X for the extrinsic Xase complex. J Biol Chem. 2000;275:28826–28833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

