Charged residues in the H-NS linker drive DNA binding and gene silencing in single cells
- PMID: 29109287
- PMCID: PMC5703333
- DOI: 10.1073/pnas.1716721114
Charged residues in the H-NS linker drive DNA binding and gene silencing in single cells
Abstract
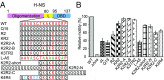
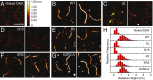
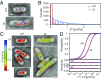
Nucleoid-associated proteins (NAPs) facilitate chromosome organization in bacteria, but the precise mechanism remains elusive. H-NS is a NAP that also plays a major role in silencing pathogen genes. We used genetics, single-particle tracking in live cells, superresolution microscopy, atomic force microscopy, and molecular dynamics simulations to examine H-NS/DNA interactions in single cells. We discovered a role for the unstructured linker region connecting the N-terminal oligomerization and C-terminal DNA binding domains. In the present work we demonstrate that linker amino acids promote engagement with DNA. In the absence of linker contacts, H-NS binding is significantly reduced, although no change in chromosome compaction is observed. H-NS is not localized to two distinct foci; rather, it is scattered all around the nucleoid. The linker makes DNA contacts that are required for gene silencing, while chromosome compaction does not appear to be an important H-NS function.
Keywords: H-NS; atomic force microscopy; nucleoid-associated proteins; single-particle tracking; superresolution microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Prajapat MK, Saini S. Interplay between Fur and HNS in controlling virulence gene expression in Salmonella typhimurium. Comput Biol Med. 2012;42:1133–1140. - PubMed
-
- Navarre WW, et al. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

