An RNA structure-mediated, posttranscriptional model of human α-1-antitrypsin expression
- PMID: 29109288
- PMCID: PMC5703279
- DOI: 10.1073/pnas.1706539114
An RNA structure-mediated, posttranscriptional model of human α-1-antitrypsin expression
Abstract
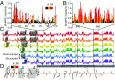
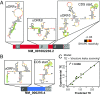
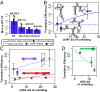
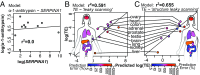
Chronic obstructive pulmonary disease (COPD) affects over 65 million individuals worldwide, where α-1-antitrypsin deficiency is a major genetic cause of the disease. The α-1-antitrypsin gene, SERPINA1, expresses an exceptional number of mRNA isoforms generated entirely by alternative splicing in the 5'-untranslated region (5'-UTR). Although all SERPINA1 mRNAs encode exactly the same protein, expression levels of the individual mRNAs vary substantially in different human tissues. We hypothesize that these transcripts behave unequally due to a posttranscriptional regulatory program governed by their distinct 5'-UTRs and that this regulation ultimately determines α-1-antitrypsin expression. Using whole-transcript selective 2'-hydroxyl acylation by primer extension (SHAPE) chemical probing, we show that splicing yields distinct local 5'-UTR secondary structures in SERPINA1 transcripts. Splicing in the 5'-UTR also changes the inclusion of long upstream ORFs (uORFs). We demonstrate that disrupting the uORFs results in markedly increased translation efficiencies in luciferase reporter assays. These uORF-dependent changes suggest that α-1-antitrypsin protein expression levels are controlled at the posttranscriptional level. A leaky-scanning model of translation based on Kozak translation initiation sequences alone does not adequately explain our quantitative expression data. However, when we incorporate the experimentally derived RNA structure data, the model accurately predicts translation efficiencies in reporter assays and improves α-1-antitrypsin expression prediction in primary human tissues. Our results reveal that RNA structure governs a complex posttranscriptional regulatory program of α-1-antitrypsin expression. Crucially, these findings describe a mechanism by which genetic alterations in noncoding gene regions may result in α-1-antitrypsin deficiency.
Keywords: RNA secondary structure; SERPINA1; translation efficiency; uORFs; α-1-antitrypsin deficiency.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Crystal RG. The alpha 1-antitrypsin gene and its deficiency states. Trends Genet. 1989;5:411–417. - PubMed
-
- Eden E, et al. Atopy, asthma, and emphysema in patients with severe alpha-1-antitrypysin deficiency. Am J Respir Crit Care Med. 1997;156:68–74. - PubMed
-
- Mahadeva R, Gaillard M, Pillay V, Halkas A, Lomas D. Characterization of a new variant of alpha(1)-antitrypsin E(Johannesburg) (H15N) in association with asthma. Hum Mutat. 2001;17:156. - PubMed
-
- Chappell S, et al. Cryptic haplotypes of SERPINA1 confer susceptibility to chronic obstructive pulmonary disease. Hum Mutat. 2006;27:103–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

