Structural basis for receptor recognition of pollen tube attraction peptides
- PMID: 29109411
- PMCID: PMC5673899
- DOI: 10.1038/s41467-017-01323-8
Structural basis for receptor recognition of pollen tube attraction peptides
Abstract
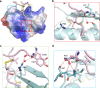
Transportation of the immobile sperms directed by pollen tubes to the ovule-enclosed female gametophytes is important for plant sexual reproduction. The defensin-like (DEFL) cysteine-rich peptides (CRPs) LUREs play an essential role in pollen tube attraction to the ovule, though their receptors still remain controversial. Here we provide several lines of biochemical evidence showing that the extracellular domain of the leucine-rich repeat receptor kinase (LRR-RK) PRK6 from Arabidopsis thaliana directly interacts with AtLURE1 peptides. Structural study reveals that a C-terminal loop of the LRR domain (AtPRK6LRR) is responsible for recognition of AtLURE1.2, mediated by a set of residues largely conserved among PRK6 homologs from Arabidopsis lyrata and Capsella rubella, supported by in vitro mutagenesis and semi-in-vivo pollen tube growth assays. Our study provides evidence showing that PRK6 functions as a receptor of the LURE peptides in A. thaliana and reveals a unique ligand recognition mechanism of LRR-RKs.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Higashiyama T, Hamamura Y. Gametophytic pollen tube guidance. Sex Plant Reprod. 2008;21:17–26. doi: 10.1007/s00497-007-0064-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

