A bacterial pioneer produces cellulase complexes that persist through community succession
- PMID: 29109478
- PMCID: PMC6794216
- DOI: 10.1038/s41564-017-0052-z
A bacterial pioneer produces cellulase complexes that persist through community succession
Abstract
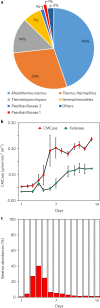
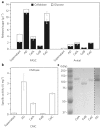
Cultivation of microbial consortia provides low-complexity communities that can serve as tractable models to understand community dynamics. Time-resolved metagenomics demonstrated that an aerobic cellulolytic consortium cultivated from compost exhibited community dynamics consistent with the definition of an endogenous heterotrophic succession. The genome of the proposed pioneer population, 'Candidatus Reconcilibacillus cellulovorans', possessed a gene cluster containing multidomain glycoside hydrolases (GHs). Purification of the soluble cellulase activity from a 300litre cultivation of this consortium revealed that ~70% of the activity arose from the 'Ca. Reconcilibacillus cellulovorans' multidomain GHs assembled into cellulase complexes through glycosylation. These remarkably stable complexes have supramolecular structures for enzymatic cellulose hydrolysis that are distinct from cellulosomes. The persistence of these complexes during cultivation indicates that they may be active through multiple cultivations of this consortium and act as public goods that sustain the community. The provision of extracellular GHs as public goods may influence microbial community dynamics in native biomass-deconstructing communities relevant to agriculture, human health and biotechnology.
Conflict of interest statement
S.K., E.D., J.H., J.M.G. and S.W.S. are inventors on a patent application related to this work (PCT/US2016/063198). The remaining authors declare no competing financial interests.
Figures

Similar articles
-
Community dynamics of cellulose-adapted thermophilic bacterial consortia.Environ Microbiol. 2013 Sep;15(9):2573-87. doi: 10.1111/1462-2920.12159. Epub 2013 Jun 13. Environ Microbiol. 2013. PMID: 23763762
-
Cellulolytic microbes in the Yanbaru, a subtropical rainforest with an endemic biota on Okinawa Island, Japan.Biosci Biotechnol Biochem. 2012;76(5):906-11. doi: 10.1271/bbb.110881. Epub 2012 May 7. Biosci Biotechnol Biochem. 2012. PMID: 22738957
-
Comparative Community Proteomics Demonstrates the Unexpected Importance of Actinobacterial Glycoside Hydrolase Family 12 Protein for Crystalline Cellulose Hydrolysis.mBio. 2016 Aug 23;7(4):e01106-16. doi: 10.1128/mBio.01106-16. mBio. 2016. PMID: 27555310 Free PMC article.
-
Driving biomass breakdown through engineered cellulosomes.Bioengineered. 2015;6(4):204-8. doi: 10.1080/21655979.2015.1060379. Bioengineered. 2015. PMID: 26068180 Free PMC article. Review.
-
The cellulosome--a treasure-trove for biotechnology.Trends Biotechnol. 1994 Sep;12(9):379-86. doi: 10.1016/0167-7799(94)90039-6. Trends Biotechnol. 1994. PMID: 7765191 Review.
Cited by
-
Xylanolytic Extremozymes Retrieved From Environmental Metagenomes: Characteristics, Genetic Engineering, and Applications.Front Microbiol. 2020 Sep 17;11:551109. doi: 10.3389/fmicb.2020.551109. eCollection 2020. Front Microbiol. 2020. PMID: 33042057 Free PMC article. Review.
-
Disturbance-diversity relationships of microbial communities change based on growth substrate.mSystems. 2024 Feb 20;9(2):e0088723. doi: 10.1128/msystems.00887-23. Epub 2024 Jan 23. mSystems. 2024. PMID: 38259105 Free PMC article.
-
Bacillales: From Taxonomy to Biotechnological and Industrial Perspectives.Microorganisms. 2022 Nov 28;10(12):2355. doi: 10.3390/microorganisms10122355. Microorganisms. 2022. PMID: 36557608 Free PMC article. Review.
-
Long-Term Cellulose Enrichment Selects for Highly Cellulolytic Consortia and Competition for Public Goods.mSystems. 2022 Apr 26;7(2):e0151921. doi: 10.1128/msystems.01519-21. Epub 2022 Mar 8. mSystems. 2022. PMID: 35258341 Free PMC article.
-
Development and characterization of stable anaerobic thermophilic methanogenic microbiomes fermenting switchgrass at decreasing residence times.Biotechnol Biofuels. 2018 Sep 6;11:243. doi: 10.1186/s13068-018-1238-1. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 30202438 Free PMC article.
References
-
- Pankratov TA, Ivanova AO, Dedysh SN, Liesack W. Bacterial populations and environmental factors controlling cellulose degradation in an acidic sphagnum peat. Environ. Microbiol. 2011;13:1800–1814. - PubMed
-
- Hanreich A, et al. Metagenome and metaproteome analyses of microbial communities in mesophilic biogas-producing anaerobic batch fermentations indicate concerted plant carbohydrate degradation. Syst. Appl. Microbiol. 2013;36:330–338. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

