Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals
- PMID: 29109537
- PMCID: PMC5674049
- DOI: 10.1038/s41598-017-15163-5
Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals
Abstract
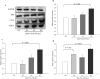
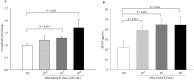
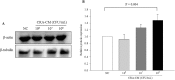
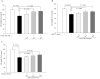
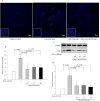
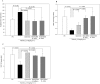
Recent evidence shows that the gut microbiota has an important role in gut-brain crosstalk and is linked to neuronal disorders. The aim of this study was to investigate the effects of intestinal Ruminococcus albus with probiotic potential on neuroprotection in oxidatively stressed SH-SY5Y neuroblastoma cells and animals. To investigate these effects, conditioned medium was prepared using Caco-2 cells cultured with heat-killed R. albus (CRA-CM). Caco-2 cells cultured with heat-killed R. albus showed increased BDNF expression and BDNF protein levels increased in CRA-CM. CRA-CM up-regulated the protein expression levels of SRF, C-fos and CDK2. In addition, CRA-CM protected SH-SY5Y cells from H2O2-induced cell death. CRA-CM significantly decreased the Bax/Bcl-2 ratio in oxidatively stressed SH-SY5Y cells. Animal experiments showed that oral administration of heat-killed R. albus for 15 days attenuated the oxidative stress induced by sodium arsenate. Treatment with heat-killed R. albus reduced the level of ROS, and the levels of SOD and GSH increased in oxidatively stressed brains. In conclusion, the secretome prepared from Caco-2 cells cultured with heat-killed R. albus might promote neuronal proliferation through the activation of cell proliferation-related proteins, and heat-killed R. albus protects neurons from oxidative damage by reducing ROS levels and increasing SOD and GSH levels.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Ye M, Kim M, Bae H. Neuro-protective effects of Ligustri Fructus by suppression of oxidative stress in mouse model of Parkinson’s disease. Oriental Pharmacy and Experimental Medicine. 2016;16:123–129. doi: 10.1007/s13596-016-0223-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

