Actors and Factors in the Resolution of Intestinal Inflammation: Lipid Mediators As a New Approach to Therapy in Inflammatory Bowel Diseases
- PMID: 29109724
- PMCID: PMC5660440
- DOI: 10.3389/fimmu.2017.01331
Actors and Factors in the Resolution of Intestinal Inflammation: Lipid Mediators As a New Approach to Therapy in Inflammatory Bowel Diseases
Abstract
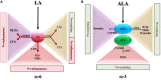
In the last few decades, the pathogenesis of inflammatory bowel disease (IBD) in genetically predisposed subjects susceptible to specific environmental factors has been attributed to disturbance of both the immune and non-immune system and/or to the imbalanced interactions with microbes. However, increasing evidences support the idea that defects in pro-resolving pathways might strongly contribute to IBD onset. The resolution of inflammation is now recognized as a dynamic event coordinated by specialized pro-resolving lipid mediators (LMs), which dampen inflammation-sustaining events, such as angiogenesis, release of pro-inflammatory cytokines, clearance of apoptotic cells, and microorganisms. Among these pro-resolving molecules, those derived from essential polyunsaturated fatty acids (PUFAs) have been shown to induce favorable effects on a plethora of human inflammatory disorders, including IBD. Here, we offer a summary of mechanisms involving both cellular and molecular components of the immune response and underlying the anti-inflammatory and pro-resolving properties of PUFAs and their derivatives in the gut, focusing on both ω-3 and ω-6 LMs. These fatty acids may influence IBD progression by: reducing neutrophil transmigration across the intestinal vasculature and the epithelium, preventing the release of pro-inflammatory cytokines and the up-regulation of adhesion molecules, and finally by promoting the production of other pro-resolving molecules. We also discuss the numerous attempts in using pro-resolving PUFAs to ameliorate intestinal inflammation, both in patients with IBD and mouse models. Although their effects in reducing inflammation is incontestable, results from previous works describing the effects of PUFA administration to prevent or treat IBD are controversial. Therefore, more efforts are needed not only to identify and explain the physiological functions of PUFAs in the gut, but also to unveil novel biosynthetic pathways of these pro-resolving LMs that may be dysregulated in these gut-related disorders. We suppose that either PUFAs or new medications specifically promoting resolution-regulating mediators and pathways will be much better tolerated by patients with IBD, with the advantage of avoiding immune suppression.
Keywords: inflammatory bowel disease; mucosal inflammation; pathogenesis; polyunsatured fatty acids; pro-resolving lipid mediators; resolution of inflammation; tissue homeostasis.
Figures

References
-
- Viggiano D, Ianiro G, Vanella G, Bibbò S, Bruno G, Simeone G, et al. Gut barrier in health and disease: focus on childhood. Eur Rev Med Pharmacol Sci (2015) 19:1077–85. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

