A study of harmful drug-drug interactions due to polypharmacy in hospitalized patients in Goa Medical College
- PMID: 29109936
- PMCID: PMC5654218
- DOI: 10.4103/picr.PICR_132_16
A study of harmful drug-drug interactions due to polypharmacy in hospitalized patients in Goa Medical College
Abstract
Introduction: Concomitant use of multiple drugs is often indicated to manage comorbid conditions and enhance efficacy. Such concomitant use of multiple drugs (five or more drugs) has been defined as "polypharmacy." Polypharmacy has been associated with adverse consequences such as greater healthcare costs, increased risk of adverse drug events, drug-drug interactions (DDIs), medication nonadherence, reduced functional capacity, and multiple geriatric syndromes. This study evaluated number of potential harmful DDIs due to polypharmacy.
Materials and methods: A prospective, cross-sectional, observational study was performed from July 2011 to June 2012. Approval was obtained from the Institutional Ethics Committee, Goa Medical College. Drug interactions were identified using a computerized DDI database system Lexi-Comp version: 2.4.1. Quantitative data analysis was done by the SPSS for Windows version 17.0.
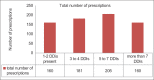
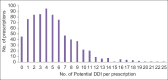
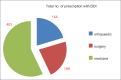
Results: Seven hundred and fifty-one out of 5424 (13.85%) prescriptions were observed to have polypharmacy with highest rates observed in the Department of Medicine. The median age of patients was 55.60 ± 13.86 (range 10-108 years). A total number of drugs per prescription ranged from minimum of 5 to maximum of 16 drugs, with an average of 7.96 ± 1.75. A large number of 596 prescriptions contained 6-9 drugs per prescription. Drugs involved in potential DDIs in our study included aspirin, antacids, beta-blockers, 3-hydroxy-3-methylglutaryl-coenzyme reductase inhibitors, calcium channel blockers, angiotensin-converting enzyme inhibitors, ondansetron, and H2 blockers.
Conclusion: Patients taking multiple medications experience unique pharmacotherapy. Personalized drug prescribing strategies and close monitoring of patients taking drugs with potential DDIs are keys to optimal therapeutic result.
Keywords: Adverse effect; drug–drug interactions; polypharmacy; super-polypharmacy.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Ruiz B, García M, Aguirre U, Aguirre C. Factors predicting hospital readmissions related to adverse drug reactions. Eur J Clin Pharmacol. 2008;64:715–22. - PubMed
-
- Kafeel H, Rukh R, Qamar H, Bawanty J, Jamshed M, Sheikh R, et al. Possibility of drug-drug interaction in prescription dispensed by community and hospital pharmacy. Pharmacology & Pharmacy. 2014;5:401–7.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous