Hemodynamic and Pathologic Characterization of the TASK-1-/- Mouse Does Not Demonstrate Pulmonary Hypertension
- PMID: 29109948
- PMCID: PMC5660113
- DOI: 10.3389/fmed.2017.00177
Hemodynamic and Pathologic Characterization of the TASK-1-/- Mouse Does Not Demonstrate Pulmonary Hypertension
Abstract
Introduction: Pulmonary hypertension (PH) carries significant associated morbidity and mortality and the underlying molecular mechanisms of PH are not well understood. Loss-of-function mutations in TASK-1 potassium channels are associated with PH in humans. Although TASK-1 has been considered in the development of PH for over a decade, characterization of TASK-1 knockout mice has been limited to in vitro studies or in vivo studies in room air at isolated time points. The purpose of this study was twofold. First, we sought to determine if TASK-/- male and female mice developed PH over the span of one year. Second, we sought to determine the effect of chronic hypoxia, a stimulus for PH, and its recovery on PH in TASK-1-/- mice.
Methods: We measured right ventricular systolic pressure (RVSP) and vascular remodeling in male and female C57BL/6 WT and TASK-1-/- mice at separate time points: 20-24 weeks and 1 year of age. Additionally, we measured RVSP and vascular remodeling in TASK-1-/- and wild-type mice between 13 and 16 weeks of age exposed to 10% hypoxia for 3 weeks followed by recovery to room air conditions for an additional 6 weeks.
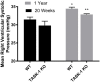
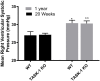
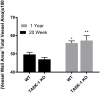
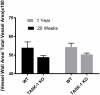
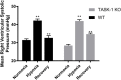
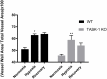
Results: RVSP was similar between WT and TASK-/- mice. Male and female WT and TASK-1-/- mice all demonstrated age-related increases in RVSP, which correlated to age-related vascular remodeling in male mice but not in female mice. Male TASK-1-/- and WT mice exposed to chronic hypoxia demonstrated increased RVSP, which decreased following room air recovery. WT and TASK-1-/- male mice demonstrated vascular remodeling upon exposure to hypoxia that persisted in room air recovery.
Conclusion: Female and male TASK-1-/- mice do not develop hemodynamic or vascular evidence for PH, but RVSP rises in an age-dependent manner independent of genotype. TASK-1-/- and WT male mice develop hypoxia-induced elevations in RVSP that decrease to baseline after recovery in room air. TASK-1-/- and WT male mice demonstrate vascular remodeling after exposure to hypoxia that persists despite recovery to room air conditions and does not correlate with RVSP normalization.
Keywords: KCNK3; TASK-1; potassium channels; pulmonary hypertension; right ventricular systolic pressures.
Figures
References
-
- Galiè N, Humbert M, Vachiery J, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J (2016) 37(1):67–119.10.1093/eurheartj/ehv317 - DOI - PubMed
-
- Olschewski A. Targeting TASK-1 channels as a therapeutic approach. In: Yuan JJ, Ward J, editors. Advances in Experimental Medicine and Biology. (Vol. 661), Totowa, NJ: Humana Press; (2009). p. 459–73. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

