TRIM proteins in blood cancers
- PMID: 29110249
- PMCID: PMC5842186
- DOI: 10.1007/s12079-017-0423-5
TRIM proteins in blood cancers
Abstract
Post-translational modification of proteins with ubiquitin plays a central role in regulating numerous cellular processes. E3 ligases determine the specificity of ubiquitination by mediating the transfer of ubiquitin to substrate proteins. The family of tripartite motif (TRIM) proteins make up one of the largest subfamilies of E3 ligases. Accumulating evidence suggests that dysregulation of TRIM proteins is associated with a variety of diseases. In this review we focus on the involvement of TRIM proteins in blood cancers.
Keywords: E3 ligase; Leukaemia; Lymphoma; Multiple myeloma; TRIM proteins; Ubiquitin.
Figures
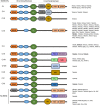
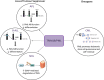
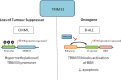
References
-
- Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, Faderl S, O'Brien S, Wierda W, Pierce S, Brandt M, McCue D, Luthra R, Patel K, Kornblau S, Kadia T, Daver N, DiNardo C, Jain N, Verstovsek S, Ferrajoli A, Andreeff M, Konopleva M, Estrov Z, Foudray M, McCue D, Cortes J, Ravandi F. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129:1275–1283. doi: 10.1182/blood-2016-09-736686. - DOI - PMC - PubMed
-
- Alfonso A, Montalban-Bravo G, Takahashi K, Jabbour EJ, Kadia T, Ravandi F, Cortes J, Estrov Z, Borthakur G, Pemmaraju N, Konopleva M, Bueso-Ramos C, Pierce S, Kantarjian H, Garcia-Manero G. Natural history of chronic myelomonocytic leukemia treated with hypomethylating agents. Am J Hematol. 2017;92:599–606. doi: 10.1002/ajh.24735. - DOI - PMC - PubMed
-
- Aucagne R, Droin N, Paggetti J, Lagrange B, Largeot A, Hammann A, Bataille A, Martin L, Yan KP, Fenaux P, Losson R, Solary E, Bastie JN, Delva L. Transcription intermediary factor 1gamma is a tumor suppressor in mouse and human chronic myelomonocytic leukemia. J Clin Invest. 2011;121:2361–2370. doi: 10.1172/JCI45213. - DOI - PMC - PubMed
-
- Bai X, Kim J, Yang Z, Jurynec MJ, Akie TE, Lee J, LeBlanc J, Sessa A, Jiang H, DiBiase A, Zhou Y, Grunwald DJ, Lin S, Cantor AB, Orkin SH, Zon LI. TIF1gamma controls erythroid cell fate by regulating transcription elongation. Cell. 2010;142:133–143. doi: 10.1016/j.cell.2010.05.028. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

