Studies of cortical connectivity using optical circuit mapping methods
- PMID: 29110301
- PMCID: PMC5767689
- DOI: 10.1113/JP273463
Studies of cortical connectivity using optical circuit mapping methods
Abstract
An important consideration when probing the function of any neuron is to uncover the source of synaptic input onto the cell, its intrinsic physiology and efferent targets. Over the years, electrophysiological approaches have generated considerable insight into these properties in a variety of cortical neuronal subtypes and circuits. However, as researchers explore neuronal function in greater detail, they are increasingly turning to optical techniques to bridge the gap between local network interactions and behaviour. The application of optical methods has increased dramatically over the past decade, spurred on by the optogenetic revolution. In this review, we provide an account of recent innovations, providing researchers with a primer detailing circuit mapping strategies in the cerebral cortex. We will focus on technical aspects of performing neurotransmitter uncaging and channelrhodopsin-assisted circuit mapping, with the aim of identifying common pitfalls that can negatively influence the collection of reliable data.
Keywords: cerebral cortex; glutamate uncaging; neural circuits; optogenetics.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures
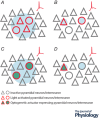
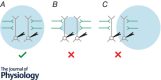
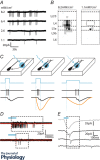
References
-
- Anastasiades PG & Butt SJ (2011). Decoding the transcriptional basis for GABAergic interneuron diversity in the mouse neocortex. Eur J Neurosci 34, 1542–1552. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

