Anti-migraine effect of ∆9-tetrahydrocannabinol in the female rat
- PMID: 29111112
- PMCID: PMC5742305
- DOI: 10.1016/j.ejphar.2017.10.054
Anti-migraine effect of ∆9-tetrahydrocannabinol in the female rat
Abstract
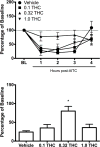
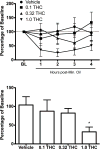
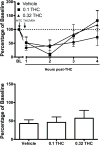
Current anti-migraine treatments have limited efficacy and many side effects. Although anecdotal evidence suggests that marijuana is useful for migraine, this hypothesis has not been tested in a controlled experiment. Thus, the present study tested whether administration of ∆9-tetrahydrocannabinol (THC) produces anti-migraine effects in the female rat. Microinjection of the TRPA1 agonist allyl isothiocyanate (AITC) onto the dura mater produced migraine-like pain for 3h as measured by depression of home cage wheel running. Concurrent systemic administration of 0.32 but not 0.1mg/kg of THC prevented AITC-induced depression of wheel running. However, 0.32mg/kg was ineffective when administered 90min after AITC. Administration of a higher dose of THC (1.0mg/kg) depressed wheel running whether rats were injected with AITC or not. Administration of a CB1, but not a CB2, receptor antagonist attenuated the anti-migraine effect of THC. These data suggest that: 1) THC reduces migraine-like pain when administered at the right dose (0.32mg/kg) and time (immediately after AITC); 2) THC's anti-migraine effect is mediated by CB1 receptors; and 3) Wheel running is an effective method to assess migraine treatments because only treatments producing antinociception without disruptive side effects will restore normal activity. These findings support anecdotal evidence for the use of cannabinoids as a treatment for migraine in humans and implicate the CB1 receptor as a therapeutic target for migraine.
Keywords: Antinociception; Headache; Marijuana; Pain-depressed behavior; Wheel running.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
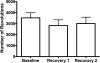


Similar articles
-
Medication overuse headache following repeated morphine, but not [INCREMENT]9-tetrahydrocannabinol administration in the female rat.Behav Pharmacol. 2018 Aug;29(5):469-472. doi: 10.1097/FBP.0000000000000382. Behav Pharmacol. 2018. PMID: 29462111 Free PMC article.
-
Depression of home cage wheel running: a reliable and clinically relevant method to assess migraine pain in rats.J Headache Pain. 2017 Dec;18(1):5. doi: 10.1186/s10194-017-0721-6. Epub 2017 Jan 13. J Headache Pain. 2017. PMID: 28091820 Free PMC article.
-
Tetrahydrocannabinol (THC) Exacerbates Inflammatory Bowel Disease in Adolescent and Adult Female Rats.J Pain. 2021 Sep;22(9):1040-1047. doi: 10.1016/j.jpain.2021.02.014. Epub 2021 Mar 13. J Pain. 2021. PMID: 33727159
-
Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: The unique susceptibility of the prepubescent animal.Neurotoxicol Teratol. 2016 Nov-Dec;58:88-100. doi: 10.1016/j.ntt.2016.02.005. Epub 2016 Feb 16. Neurotoxicol Teratol. 2016. PMID: 26898326 Review.
-
New perspectives in the studies on endocannabinoid and cannabis: abnormal behaviors associate with CB1 cannabinoid receptor and development of therapeutic application.J Pharmacol Sci. 2004 Dec;96(4):362-6. doi: 10.1254/jphs.fmj04003x2. Epub 2004 Dec 10. J Pharmacol Sci. 2004. PMID: 15599103 Review.
Cited by
-
Interplay between cannabinoids and the neuroimmune system in migraine.J Headache Pain. 2024 Oct 16;25(1):178. doi: 10.1186/s10194-024-01883-3. J Headache Pain. 2024. PMID: 39407099 Free PMC article. Review.
-
Emerging Role of (Endo)Cannabinoids in Migraine.Front Pharmacol. 2018 Apr 24;9:420. doi: 10.3389/fphar.2018.00420. eCollection 2018. Front Pharmacol. 2018. PMID: 29740328 Free PMC article. Review.
-
Natural Products Derived from Cannabis sativa for Pain Management.Handb Exp Pharmacol. 2025;287:239-263. doi: 10.1007/164_2024_710. Handb Exp Pharmacol. 2025. PMID: 38509238 Review.
-
Use of home cage wheel running to assess the behavioural effects of administering a mu/delta opioid receptor heterodimer antagonist for spontaneous morphine withdrawal in the rat.Behav Brain Res. 2021 Jan 15;397:112953. doi: 10.1016/j.bbr.2020.112953. Epub 2020 Oct 6. Behav Brain Res. 2021. PMID: 33031872 Free PMC article.
-
Bridging Gaps in Migraine Management: A Comprehensive Review of Conventional Treatments, Natural Supplements, Complementary Therapies, and Lifestyle Modifications.Pharmaceuticals (Basel). 2025 Jan 22;18(2):139. doi: 10.3390/ph18020139. Pharmaceuticals (Basel). 2025. PMID: 40005953 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

