Density dependent re-tuning of autoreactive T cells alleviates their pathogenicity in a lymphopenic environment
- PMID: 29111199
- PMCID: PMC5687833
- DOI: 10.1016/j.imlet.2017.10.005
Density dependent re-tuning of autoreactive T cells alleviates their pathogenicity in a lymphopenic environment
Abstract
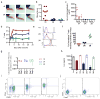
Peripheral T cell tolerance is challenging to induce in partially lymphopenic hosts and this is relevant for clinical situations involving transplant tolerance. While the shortage of regulatory cells is thought to be one reason for this, T cell-intrinsic tolerance processes such as anergy are also poorly triggered in such hosts. In order to understand the latter, we used a T cell deficient mouse model system where adoptively transferred autoreactive T cells are significantly tolerized in a cell intrinsic fashion, without differentiation to regulatory T cells. Intriguingly these T cells often retain sufficient effector functions to trigger autoimmune pathology. Here we find that the high population density of the autoreactive T cells that accumulated in such a host limits the progression of the cell-intrinsic tolerance process in T cells. Accordingly, reducing the cell density during a second transfer allowed T cells to further tune down their responsiveness to antigenic stimulation. The retuning of T cells was reflected by a loss of the T cell's abilities to proliferate, produces cytokines or help B cells. We further suggest, based on altering the levels of chronic antigen using miniosmotic pumps, that the effects of cell-density on T cell re-tuning may reflect the effective changes in the antigen dose perceived by individual T cells. This could proportionally elicit more negative feedback downstream of the TCR. Consistent with this, the retuned T cells showed signaling defects both proximal and distal to the TCR. Therefore, similar to the immunogenic activation of T cells, cell-intrinsic T cell tolerance may also involve a quantitative and progressive process of tuning down its antigen-responsiveness. The progress of such tuning seems to be stabilized at multiple intermediate stages by factors such as cell density, rather than just absolute antigen levels.
Copyright © 2017 European Federation of Immunological Societies. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions.Proc Natl Acad Sci U S A. 2008 Dec 9;105(49):19414-9. doi: 10.1073/pnas.0807743105. Epub 2008 Nov 25. Proc Natl Acad Sci U S A. 2008. PMID: 19033460 Free PMC article.
-
Effects and regulation of autoreactive CD8+ T cells in a transgenic mouse model of autoimmune hepatitis.Gastroenterology. 2010 Sep;139(3):975-86, 986.e1-3. doi: 10.1053/j.gastro.2010.05.075. Epub 2010 Jun 2. Gastroenterology. 2010. PMID: 20639127
-
Regulation of cytokine-driven functional differentiation of CD8 T cells by suppressor of cytokine signaling 1 controls autoimmunity and preserves their proliferative capacity toward foreign antigens.J Immunol. 2010 Jul 1;185(1):357-66. doi: 10.4049/jimmunol.1000066. Epub 2010 Jun 2. J Immunol. 2010. PMID: 20519645
-
Antigen-nonspecific activation of CD8+ T lymphocytes by cytokines: relevance to immunity, autoimmunity, and cancer.Arch Immunol Ther Exp (Warsz). 2008 Sep-Oct;56(5):311-23. doi: 10.1007/s00005-008-0033-2. Arch Immunol Ther Exp (Warsz). 2008. PMID: 18836862 Review.
-
Tolerance to islet autoantigens in type 1 diabetes.Annu Rev Immunol. 2001;19:131-61. doi: 10.1146/annurev.immunol.19.1.131. Annu Rev Immunol. 2001. PMID: 11244033 Review.
Cited by
-
Manipulating the TCR signaling network for cellular immunotherapy: Challenges & opportunities.Mol Immunol. 2020 Jul;123:64-73. doi: 10.1016/j.molimm.2020.04.007. Epub 2020 May 15. Mol Immunol. 2020. PMID: 32422416 Free PMC article. Review.
References
-
- Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435(7042):598–604. - PubMed
-
- Singh NJ, Schwartz RH. Primer: mechanisms of immunologic tolerance. Nature Clinical Practice Rheumatology. 2006;2(1):44–52. - PubMed
-
- Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8(5):457–462. - PubMed
-
- Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Jr, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(16):8789–94. - PMC - PubMed
-
- Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transplant immunology. 2004;13(2):117–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

