Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk
- PMID: 29112070
- PMCID: PMC5690310
- DOI: 10.1097/QAD.0000000000001675
Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk
Abstract
Objective: To compare the efficacy, safety, and impact on lipid fractions of switching from a ritonavir-boosted protease inhibitor (PI/r) to a dolutegravir (DTG) regimen.
Methods: HIV type 1-infected adults more than 50 years or with a Framingham score more than 10% were eligible if plasma HIV RNA less than 50 copies per ml for at least 24 weeks while on a PI/r regimen. Patients were randomized to switch to DTG or to remain on PI/r. Primary endpoints were: proportion maintaining HIV RNA less than 50 copies per ml and percentage change from baseline of total cholesterol at week 48.
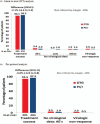
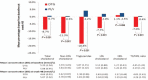
Results: In total, 415 patients (32 sites in six European countries) were randomized: 205 to DTG and 210 to continue PI/r. About 89% were men, 87% more than 50 years, 74% had a Framingham score more than 10%, with a median CD4 cell count of 617 cells per μl and suppressed viremia for a median of 5 years. At week 48, in the intent-to-treat analysis, treatment success rate was 93.1% in DTG group and 95.2% in PI/r group (difference -2.1%, 95% confidence interval -6.6 to 2.4, noninferiority demonstrated). There were four virological failures with DTG and one with PI/r with no emergent resistance mutations. There was no significant difference in severe adverse events or grade 3 or 4 adverse events or treatment modifying adverse events. Total cholesterol and other lipid fractions (except high-density lipoprotein cholesterol) improved significantly (P < 0.001) in the DTG group regardless of PI/r at baseline.
Conclusion: Switching to a DTG regimen in virologically suppressed HIV type 1 patients with high cardiovascular disease risk was noninferior, and significantly improved lipid profiles.
Figures
Comment in
-
Reducing medical comorbidities associated with long-term HIV infection: beyond optimizing antiretroviral therapy regimens.AIDS. 2017 Nov 28;31(18):2547-2549. doi: 10.1097/QAD.0000000000001677. AIDS. 2017. PMID: 29120900 No abstract available.
References
-
- Llibre JM, Walmsley S, Gatell JM. Backbones versus core agents in initial ART regimens: one game, two players. J Antimicrob Chemother 2016; 71:856–861. - PubMed
-
- Cohn J, Bekker LG, Bygrave H, Calmy A. Hit me with your best shot: dolutegravir: a space in the next WHO guidelines?. AIDS 2015; 29:2067–2070. - PubMed
-
- Martinez E, Larrousse M, Llibre JM, Gutierrez F, Saumoy M, Antela A, et al. SPIRAL Study Group. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS 2010; 24:1697–1707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous