Longitudinal analysis of quality-of-life outcomes in children during treatment for acute lymphoblastic leukemia: A report from the Children's Oncology Group AALL0932 trial
- PMID: 29112230
- PMCID: PMC5808870
- DOI: 10.1002/cncr.31085
Longitudinal analysis of quality-of-life outcomes in children during treatment for acute lymphoblastic leukemia: A report from the Children's Oncology Group AALL0932 trial
Abstract
Background: Children with average-risk acute lymphoblastic leukemia (AR-ALL) face many challenges that can adversely affect their quality of life (QOL). However, to the authors' knowledge, patterns and predictors of QOL impairment during therapy have not been well characterized to date.
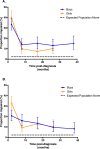
Methods: Patients with AR-ALL who were enrolled on the Children's Oncology Group AALL0932 trial were offered participation in this prospective cohort study if they were aged ≥4 years at the time of diagnosis and had an English-speaking parent. At approximately 2 months, 8 months, 17 months, 26 months, and 38 months (boys only) after diagnosis, parents completed the Pediatric Quality of Life Inventory Generic Core Scales Version 4.0 (PedsQL4.0) and McMaster Family Assessment Device instruments for QOL (physical, emotional, and social functioning) and family functioning, respectively. The proportions of individuals scoring in the impaired range (2 standard deviations below the population mean) were calculated at each time point. Longitudinal impairment patterns and predictors were examined.
Results: A total of 594 participants with AR-ALL were diagnosed at a mean age of 6.0 years (standard deviation, 1.6 years). At 2 months, a substantial proportion of participants had impaired scores for physical (36.5%; 95% confidence interval [95% CI], 32.3%-40.8%) and emotional (26.2%; 95% CI, 22.5%-30.2%) functioning compared with population norms of 2.3%. These elevations persisted at 26 months. Emotional impairment at 2 months (odds ratio, 3.4; 95% CI, 1.5-7.7) was found to significantly predict emotional impairment at 26 months. In repeated measures analysis with multivariate modeling, unhealthy family functioning (odds ratio, 1.5; 95% CI, 1.1-2.1) significantly predicted emotional impairment controlling for age and sex. QOL outcomes were similar between sexes at the end of therapy (26 months for girls and 38 months for boys).
Conclusions: Many children with AR-ALL experience physical and emotional functioning impairment that begins early in treatment and persists. Early screening may identify high-risk patients who might benefit from family-based interventions. Cancer 2018;124:571-9. © 2017 American Cancer Society.
Keywords: acute lymphoblastic leukemia; emotional functioning; family functioning; health-related quality of life; pediatric.
© 2017 American Cancer Society.
Conflict of interest statement
A. Angiolillo, L. Balsamo, W. Carroll, M. Devidas, S. Hunger, N. Kadan-Lottick, M. Loh, X. Lu, E. Raetz, L. Sung, N. Winick, and D. Zheng report no conflicts of interest.
Figures


References
-
- Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. [Based on the November 2012 SEER data submission, posted to the SEER web site, April 2013.] Bethesda, MD: National Cancer Institute; 2013.
-
- Sung L, Yanofsky R, Klaassen RJ, et al. Quality of life during active treatment for pediatric acute lymphoblastic leukemia. International Journal of Cancer. 2011;128:1213–1220. - PubMed
-
- van Litsenburg RR, Kunst A, Huisman J, Ket JC, Kaspers GJ, Gemke RJ. Health Status Utilities in Pediatrics A Systematic Review of Acute Lymphoblastic Leukemia. Medical Decision Making. 2013:0272989X13497263. - PubMed
-
- Pickard AS, Topfer L-A, Feeny DH. A structured review of studies on health-related quality of life and economic evaluation in pediatric acute lymphoblastic leukemia. Journal of the National Cancer Institute Monographs. 2004;2004:102–125. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

