Chemical Biology of H2S Signaling through Persulfidation
- PMID: 29112440
- PMCID: PMC6029264
- DOI: 10.1021/acs.chemrev.7b00205
Chemical Biology of H2S Signaling through Persulfidation
Abstract
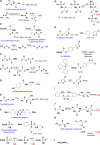
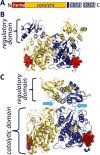
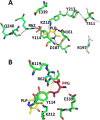
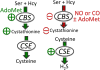
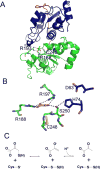
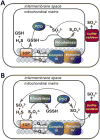
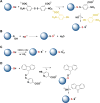
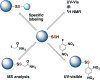
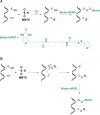
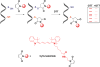
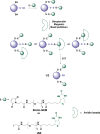
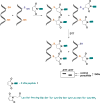
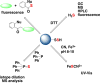
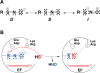
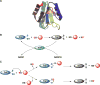
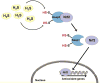
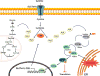
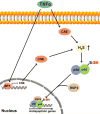
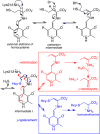
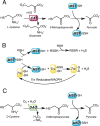
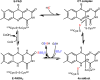
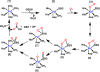
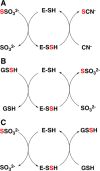
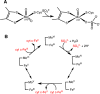
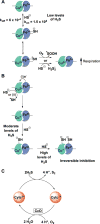
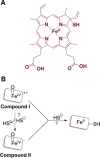
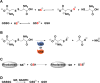
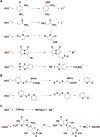
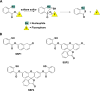
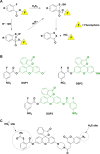
Signaling by H2S is proposed to occur via persulfidation, a posttranslational modification of cysteine residues (RSH) to persulfides (RSSH). Persulfidation provides a framework for understanding the physiological and pharmacological effects of H2S. Due to the inherent instability of persulfides, their chemistry is understudied. In this review, we discuss the biologically relevant chemistry of H2S and the enzymatic routes for its production and oxidation. We cover the chemical biology of persulfides and the chemical probes for detecting them. We conclude by discussing the roles ascribed to protein persulfidation in cell signaling pathways.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
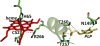
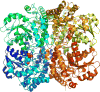
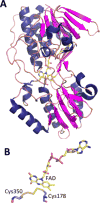

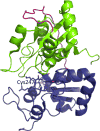


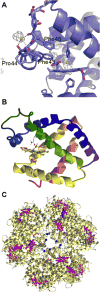
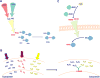










References
-
- Winogradsky S. Ueber Schwefelbakterien. Bot Zeitung. 1887;45:489–507. 513–523, 529–539, 545–559, 569–576, 585–594.
-
- Ramazzini B. Baptiftam Conzattum; Padua: 1713. De Morbis Artificum Diatriba. - PubMed
-
- Scheele CW. Chemische Abhandlung von der Luft und dem Feuer (Chemical treatise on air and fire) Magnus Swederus; Upsala, Sweden: 1777.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

