Severe Influenza Is Characterized by Prolonged Immune Activation: Results From the SHIVERS Cohort Study
- PMID: 29112724
- PMCID: PMC7335675
- DOI: 10.1093/infdis/jix571
Severe Influenza Is Characterized by Prolonged Immune Activation: Results From the SHIVERS Cohort Study
Abstract
Background: The immunologic factors underlying severe influenza are poorly understood. To address this, we compared the immune responses of influenza-confirmed hospitalized individuals with severe acute respiratory illness (SARI) to those of nonhospitalized individuals with influenza-like illness (ILI).
Methods: Peripheral blood lymphocytes were collected from 27 patients with ILI and 27 with SARI, at time of enrollment and then 2 weeks later. Innate and adaptive cellular immune responses were assessed by flow cytometry, and serum cytokine levels were assessed by a bead-based assay.
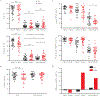
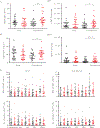
Results: During the acute phase, SARI was associated with significantly reduced numbers of circulating myeloid dendritic cells, CD192+ monocytes, and influenza virus-specific CD8+ and CD4+ T cells as compared to ILI. By the convalescent phase, however, most SARI cases displayed continued immune activation characterized by increased numbers of CD16+ monocytes and proliferating, and influenza virus-specific, CD8+ T cells as compared to ILI cases. SARI was also associated with reduced amounts of cytokines that regulate T-cell responses (ie, interleukin 4, interleukin 13, interleukin 12, interleukin 10, and tumor necrosis factor β) and hematopoiesis (interleukin 3 and granulocyte-macrophage colony-stimulating factor) but increased amounts of a proinflammatory cytokine (tumor necrosis factor α), chemotactic cytokines (MDC, MCP-1, GRO, and fractalkine), and growth-promoting cytokines (PDGFBB/AA, VEGF, and EGF) as compared to ILI.
Conclusions: Severe influenza cases showed a delay in the peripheral immune activation that likely led prolonged inflammation, compared with mild influenza cases.
Keywords: Influenza; cellular immunity; cytokine; disease severity; infection.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Conflict of interest statement
Figures



References
-
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

