Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- PMID: 29112958
- PMCID: PMC5675358
- DOI: 10.1371/journal.pmed.1002424
Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
Abstract
Background: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study has developed predictive risk scores for cardiovascular disease (CVD) and chronic kidney disease (CKD, defined as confirmed estimated glomerular filtration rate [eGFR] ≤ 60 ml/min/1.73 m2) events in HIV-positive people. We hypothesized that participants in D:A:D at high (>5%) predicted risk for both CVD and CKD would be at even greater risk for CVD and CKD events.
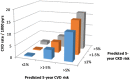
Methods and findings: We included all participants with complete risk factor (covariate) data, baseline eGFR > 60 ml/min/1.73 m2, and a confirmed (>3 months apart) eGFR < 60 ml/min/1.73 m2 thereafter to calculate CVD and CKD risk scores. We calculated CVD and CKD event rates by predicted 5-year CVD and CKD risk groups (≤1%, >1%-5%, >5%) and fitted Poisson models to assess whether CVD and CKD risk group effects were multiplicative. A total of 27,215 participants contributed 202,034 person-years of follow-up: 74% male, median (IQR) age 42 (36, 49) years, median (IQR) baseline year of follow-up 2005 (2004, 2008). D:A:D risk equations predicted 3,560 (13.1%) participants at high CVD risk, 4,996 (18.4%) participants at high CKD risk, and 1,585 (5.8%) participants at both high CKD and high CVD risk. CVD and CKD event rates by predicted risk group were multiplicative. Participants at high CVD risk had a 5.63-fold (95% CI 4.47, 7.09, p < 0.001) increase in CKD events compared to those at low risk; participants at high CKD risk had a 1.31-fold (95% CI 1.09, 1.56, p = 0.005) increase in CVD events compared to those at low risk. Participants' CVD and CKD risk groups had multiplicative predictive effects, with no evidence of an interaction (p = 0.329 and p = 0.291 for CKD and CVD, respectively). The main study limitation is the difference in the ascertainment of the clinically defined CVD endpoints and the laboratory-defined CKD endpoints.
Conclusions: We found that people at high predicted risk for both CVD and CKD have substantially greater risks for both CVD and CKD events compared with those at low predicted risk for both outcomes, and compared to those at high predicted risk for only CVD or CKD events. This suggests that CVD and CKD risk in HIV-positive persons should be assessed together. The results further encourage clinicians to prioritise addressing modifiable risks for CVD and CKD in HIV-positive people.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: MB has received research grants, paid to his institution from Merck Sharp & Dohme and Gilead Sciences. MB has also received honoraria for participation on HIV advisory boards and preparation and delivery of educational materials from Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme and ViiV Healthcare. AM has received honoraria, travel support, speaker fees and consultancy fees from ViiV, BMS, BI, Gilead, and Wragge LLC; AP received speaker fees for a 2015 conference for Gilead Sciences; ML has received unrestricted research grants, paid to his institution, from Boehringer Ingelhiem, Gilead Sciences, Merck Sharp & Dohme, Bristol-Myers Squibb, Janssen-Cilag, ViiV HealthCare; ML has also received consultancy and presentation fees from Gilead Sciences, and DSMB sitting fees from Sirtex Pty Ltd; PR through his institution has received independent scientific grant support from Gilead Sciences, Janssen Pharmaceuticals Inc, Merck & Co, Bristol-Myers Squibb and ViiV Healthcare; PR has served on a scientific advisory board for Gilead Sciences and ViiV Healthcare, as well as on a data safety monitoring committee for Janssen Pharmaceuticals Inc, and has chaired a scientific symposium by ViiV and received remuneration; CS's institution has received a grant from the D:A:D Oversight Committee for the analysis and co-ordination of the D:A:D Study; CS has also received personal fees from Gilead Sciences, ViiV Healthcare and Janssen-Cilag for the membership of Data Safety and Monitoring Boards, Advisory Boards, Speaker Panels and for the preparation of educational materials.
Figures
References
-
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301 - DOI - PubMed
-
- Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sørensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. - PubMed
-
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE. 2013;8(12):e81355 doi: 10.1371/journal.pone.0081355 - DOI - PMC - PubMed
-
- Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–56. doi: 10.1016/S2352-3018(17)30066-8 - DOI - PMC - PubMed
-
- Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous