Adjustment of the dynamic weight distribution as a sensitive parameter for diagnosis of postural alteration in a rodent model of vestibular deficit
- PMID: 29112981
- PMCID: PMC5675415
- DOI: 10.1371/journal.pone.0187472
Adjustment of the dynamic weight distribution as a sensitive parameter for diagnosis of postural alteration in a rodent model of vestibular deficit
Abstract
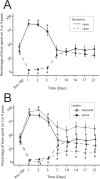
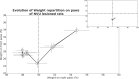
Vestibular disorders, by inducing significant posturo-locomotor and cognitive disorders, can significantly impair the most basic tasks of everyday life. Their precise diagnosis is essential to implement appropriate therapeutic countermeasures. Monitoring their evolution is also very important to validate or, on the contrary, to adapt the undertaken therapeutic actions. To date, the diagnosis methods of posturo-locomotor impairments are restricted to examinations that most often lack sensitivity and precision. In the present work we studied the alterations of the dynamic weight distribution in a rodent model of sudden and complete unilateral vestibular loss. We used a system of force sensors connected to a data analysis system to quantify in real time and in an automated way the weight bearing of the animal on the ground. We show here that sudden, unilateral, complete and permanent loss of the vestibular inputs causes a severe alteration of the dynamic ground weight distribution of vestibulo lesioned rodents. Characteristics of alterations in the dynamic weight distribution vary over time and follow the sequence of appearance and disappearance of the various symptoms that compose the vestibular syndrome. This study reveals for the first time that dynamic weight bearing is a very sensitive parameter for evaluating posturo-locomotor function impairment. Associated with more classical vestibular examinations, this paradigm can considerably enrich the methods for assessing and monitoring vestibular disorders. Systematic application of this type of evaluation to the dizzy or unstable patient could improve the detection of vestibular deficits and allow predicting better their impact on posture and walk. Thus it could also allow a better follow-up of the therapeutic approaches for rehabilitating gait and balance.
Conflict of interest statement
Figures





References
-
- Asher DL. Vestibular anatomy and physiology In: Northern JL. editor. Hearing Disorders. 2 Boston, MA: Little, Brown and Company; 1984; 195–204.
-
- Luxon LM. The anatomy and physiology of the vestibular system In: Dix MR, Hood JD editors. Vertigo. Chichester, UK: John Wiley & Sons, Ltd; 1984; 1–33.
-
- Jones SM, Jones TA, Mills KN, Gaines GC. Anatomical and physiological considerations in vestibular dysfunction and compensation. Semin Hear. 2009; 30(4): 231–241. doi: 10.1055/s-0029-1241124 - DOI - PMC - PubMed
-
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008; 31: 125–150. doi: 10.1146/annurev.neuro.31.060407.125555 - DOI - PubMed
-
- Lacour M, Tighilet B. Plastic events in the vestibular nuclei during vestibular compensation: the brain orchestration of a deafferentation code. Rest Neurol Neurosci. 2010; 27: 1–17. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

