First-Row Late Transition Metals for Catalytic Alkene Hydrofunctionalisation: Recent Advances in C-N, C-O and C-P Bond Formation
- PMID: 29113059
- PMCID: PMC6150243
- DOI: 10.3390/molecules22111901
First-Row Late Transition Metals for Catalytic Alkene Hydrofunctionalisation: Recent Advances in C-N, C-O and C-P Bond Formation
Abstract
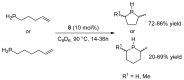
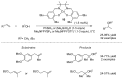
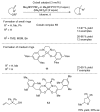
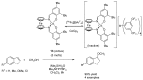
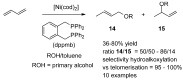
This review provides an outline of the most noteworthy achievements in the area of C-N, C-O and C-P bond formation by hydroamination, hydroalkoxylation, hydrophosphination, hydrophosphonylation or hydrophosphinylation reaction on unactivated alkenes (including 1,2- and 1,3-dienes) promoted by first-row late transition metal catalytic systems based on manganese, iron, cobalt, nickel, copper and zinc. The relevant literature from 2009 until mid-2017 has been covered.
Keywords: alcohols; amines; first-row late transition metals; hydrofunctionalisation; phosphorus compounds; unactivated alkenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

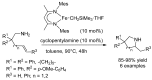



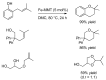
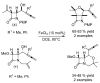



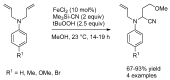
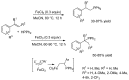
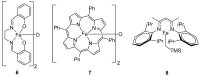
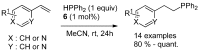

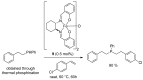

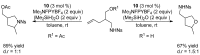
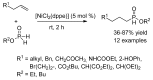





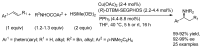



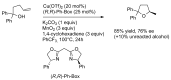




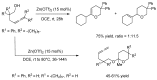
References
-
- Ananikov V.P., Tanaka M., editors. Hydrofunctionalization. Volume 43. Springer; Berlin/Heidelberg, Germany: 2013. pp. 1–325.
-
- Rodriguez-Ruiz V., Carlino R., Bezzenine-Lafollée S., Gil R., Prim D., Schulz E., Hannedouche J. Recent developments in alkene hydrofunctionalisation promoted by homogeneous catalysts based on earth abundant elements: Formation of C-N, C-O and C-P bond. Dalton Trans. 2015;44:12029–12059. doi: 10.1039/C5DT00280J. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

