The Oligomeric Form of the Escherichia coli Dps Protein Depends on the Availability of Iron Ions
- PMID: 29113077
- PMCID: PMC6150300
- DOI: 10.3390/molecules22111904
The Oligomeric Form of the Escherichia coli Dps Protein Depends on the Availability of Iron Ions
Abstract
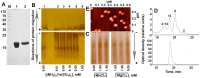
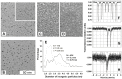
The Dps protein of Escherichia coli, which combines ferroxidase activity and the ability to bind DNA, is effectively used by bacteria to protect their genomes from damage. Both activities depend on the integrity of this multi-subunit protein, which has an inner cavity for iron oxides; however, the diversity of its oligomeric forms has only been studied fragmentarily. Here, we show that iron ions stabilize the dodecameric form of Dps. This was found by electrophoretic fractionation and size exclusion chromatography, which revealed several oligomers in highly purified protein samples and demonstrated their conversion to dodecamers in the presence of 1 mM Mohr's salt. The transmission electron microscopy data contradicted the assumption that the stabilizing effect is given by the optimal core size formed in the inner cavity of Dps. The charge state of iron ions was evaluated using Mössbauer spectroscopy, which showed the presence of Fe₃O₄, rather than the expected Fe₂O₃, in the sample. Assuming that Fe2+ can form additional inter-subunit contacts, we modeled the interaction of FeO and Fe₂O₃ with Dps, but the binding sites with putative functionality were predicted only for Fe₂O₃. The question of how the dodecameric form can be stabilized by ferric oxides is discussed.
Keywords: Dps proteins; Escherichia coli; Mössbauer spectroscopy; ferroxidase; molecular docking; oligomerization; transmission electron microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Talukder A.A., Ishihama A. Dps is a Stationary Phase-Specific Protein of Escherichia coli Nucleoid. Adv. Microbiol. 2014;4:1095–1104. doi: 10.4236/aim.2014.415120. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

