Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis
- PMID: 29113135
- PMCID: PMC5707692
- DOI: 10.3390/nu9111220
Nonalcoholic Fatty Liver Disease Is Exacerbated in High-Fat Diet-Fed Gnotobiotic Mice by Colonization with the Gut Microbiota from Patients with Nonalcoholic Steatohepatitis
Abstract
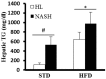
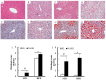
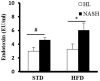
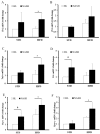
Nonalcoholic fatty liver disease (NAFLD) is a serious liver disorder associated with the accumulation of fat and inflammation. The objective of this study was to determine the gut microbiota composition that might influence the progression of NAFLD. Germ-free mice were inoculated with feces from patients with nonalcoholic steatohepatitis (NASH) or from healthy persons (HL) and then fed a standard diet (STD) or high-fat diet (HFD). We found that the epididymal fat weight, hepatic steatosis, multifocal necrosis, and inflammatory cell infiltration significantly increased in the NASH-HFD group. These findings were consistent with markedly elevated serum levels of alanine transaminase, aspartate transaminase, endotoxin, interleukin 6 (IL-6), monocyte chemotactic protein 1 (Mcp1), and hepatic triglycerides. In addition, the mRNA expression levels of Toll-like receptor 2 (Tlr2), Toll-like receptor 4 (Tlr4), tumor necrosis factor alpha (Tnf-α), Mcp1, and peroxisome proliferator-activated receptor gamma (Ppar-γ) significantly increased. Only abundant lipid accumulation and a few inflammatory reactions were observed in group HL-HFD. Relative abundance of Bacteroidetes and Firmicutes shifted in the HFD-fed mice. Furthermore, the relative abundance of Streptococcaceae was the highest in group NASH-HFD. Nevertheless, obesity-related Lactobacillaceae were significantly upregulated in HL-HFD mice. Our results revealed that the gut microbiota from NASH Patients aggravated hepatic steatosis and inflammation. These findings might partially explain the NAFLD progress distinctly was related to different compositions of gut microbiota.
Keywords: gut microbiota; high-fat diet; humanized gnotobiotic mice; nonalcoholic fatty liver disease.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Adams L.A., Lymp J.F., St. Sauver J., Sanderson S.O., Lindor K.D., Feldstein A., Angulo P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology. 2005;129:113–121. - PubMed
-
- Liang W., Menke A.L., Driessen A., Koek G.H., Lindeman J.H., Stoop R., Havekes L.M., Kleemann R., van den Hoek A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE. 2014;9:e115922. doi: 10.1371/journal.pone.0115922. - DOI - PMC - PubMed
-
- Ganz M., Bukong T.N., Csak T., Saha B., Park J.K., Ambade A., Kodys K., Szabo G. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J. Transl. Med. 2015;13:193. doi: 10.1186/s12967-015-0552-7. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

