EZH2 and histone deacetylase inhibitors induce apoptosis in triple negative breast cancer cells by differentially increasing H3 Lys27 acetylation in the BIM gene promoter and enhancers
- PMID: 29113202
- PMCID: PMC5661363
- DOI: 10.3892/ol.2017.6912
EZH2 and histone deacetylase inhibitors induce apoptosis in triple negative breast cancer cells by differentially increasing H3 Lys27 acetylation in the BIM gene promoter and enhancers
Abstract
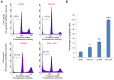
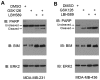
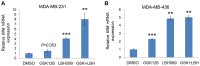
Enhancer of zeste homolog 2 (EZH2), a subunit of polycomb repressive complex 2, is a histone methyl-transferase and is considered to work cooperatively with histone deacetylases (HDACs) in the same protein complex to mediate gene transcription repression by increasing histone H3 Lys27 trimethylation (H3K27me3), in particular in the nucleosome (s). EZH2 is overexpressed in numerous types of cancer, including triple negative breast cancer (TNBC), a subtype of breast cancer, which there are no effective treatment options for. Thus, inhibition of EZH2 may be harnessed for targeted therapy of this disease. The present study demonstrated that co-treatment with an EZH2 inhibitor and a HDAC inhibitor additively induced apoptosis in two TNBC cell lines, namely MDA-MB-231 and MDA-MB-436. The increased rate of cell death was associated with an elevation of B cell lymphoma-2 like 11 (BIM) expression level, a pro-apoptotic protein at the protein and mRNA expression levels in these two cell lines. The expression of forkhead box O1 (FOXO1), a known upstream transcriptional activator of BIM, was upregulated in both cell lines by the HDAC inhibitor, and the effect was more pronounced in MDA-MB-436 cells with higher phosphorylation levels of protein kinase B, a negative regulator of FOXO1, compared with MDA-MB-231 cells. Conversely, FOXO1 expression was inhibited following treatment with the EZH2 inhibitor, suggesting that EZH2 and HDAC inhibitors induced BIM expression via a FOXO1-independent mechanism. The present study further revealed that the EZH2 inhibitor, but not the HDAC inhibitor, induced high levels of H3K27 acetylation (H3K27ac) in the BIM promoter. By contrast, compared with the effect of the EZH2 inhibitor, HDAC inhibitor treatment resulted in an increase in H3K27ac at two BIM enhancers. Collectively, the results of the present study indicated that EZH2 and HDACs act differentially on H3K27ac levels in the nucleosome at the promoter and enhancer regions of the BIM gene. Through the upregulation of BIM, co-treatment with EZH2 and HDAC inhibitors had a pronounced therapeutic effect on TNBC cells, suggesting that co-targeting EZH2 and HDAC proteins represents a viable therapeutic option for the treatment of TNBC.
Keywords: B cell lymphoma-2 like 11; chemotherapy; enhancer of zeste 2 polycomb repressive complex 2 subunit; forkhead box O1; histone H3 Lys27 acetylation; histone H3 Lys27 trimethylation; histone deacetylases; triple negative breast cancer.
Figures



References
-
- Donepudi MS, Kondapalli K, Amos SJ, Venkanteshan P. Breast cancer statistics and markers. J Cancer Res Ther. 2014;10:506–511. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
