Mitochondrion-associated protein peroxiredoxin 3 promotes benign prostatic hyperplasia through autophagy suppression and pyroptosis activation
- PMID: 29113303
- PMCID: PMC5655198
- DOI: 10.18632/oncotarget.17927
Mitochondrion-associated protein peroxiredoxin 3 promotes benign prostatic hyperplasia through autophagy suppression and pyroptosis activation
Abstract
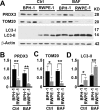
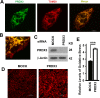
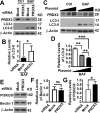
Benign prostatic hyperplasia (BPH) is one of the most common diseases in the senior men and age plays an important role in the initiation and development of BPH. Mammalian cells primarily use the autophagy-lysosome system to degrade misfolded/aggregated proteins and dysfunctional organelles such as mitochondria and suppress pyroptosis, a type of cell death that stimulates inflammatory responses and growth of other cells around. Peroxiredoxin 3 (PRDX3) is the only mitochondrion-associated member of peroxiredoxin family enzymes that exert their protective antioxidant role in cells through their peroxidase activity. We hypothesized that PRDX3 may inhibit autophagy to activate pyroptosis to induce growth of prostatic epithelial cells. Here we show that PRDX3 maintained the integrity of mitochondria and its depletion led to an enhancement of oxidative stresses. PRDX3-associated and PRDX3-free mitochondria co-existed in the same cells. PRDX3 expressed at higher levels in prostatic epithelial cells in prostate tissues from BPH patients and BPH-representative cell line than in prostate tissues from healthy donors and a cell line representing normal epithelial cells. PRDX3 suppressed autophagy flux and activated pyroptosis to induce inflammatory responses and stimulate the over-growth of prostate tissues. Therefore, higher levels of PDRX3 in prostatic epithelial cells may promote the initiation and development of BPH through autophagy inhibition and pyroptosis activation.
Keywords: BPH; LC3; autophagy; benign prostatic hyperplasia; caspase 1.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest is disclosed.
Figures

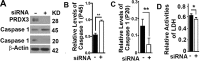
Similar articles
-
The pathogenesis of benign prostatic hyperplasia and the roles of Prdx3, oxidative stress, pyroptosis and autophagy:a review.Front Oncol. 2025 Aug 5;15:1579539. doi: 10.3389/fonc.2025.1579539. eCollection 2025. Front Oncol. 2025. PMID: 40837016 Free PMC article. Review.
-
Measurement of autophagy flux in benign prostatic hyperplasia in vitro.Prostate Int. 2020 Jun;8(2):70-77. doi: 10.1016/j.prnil.2019.11.004. Epub 2020 Feb 26. Prostate Int. 2020. PMID: 32647643 Free PMC article.
-
Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia.Prostate. 2004 Jul 1;60(2):153-9. doi: 10.1002/pros.20051. Prostate. 2004. PMID: 15162381
-
Inhibition of androgen induces autophagy in benign prostate epithelial cells.Int J Urol. 2014 Feb;21(2):195-9. doi: 10.1111/iju.12210. Epub 2013 Jul 2. Int J Urol. 2014. PMID: 23819759
-
Autophagy in benign prostatic hyperplasia: insights and therapeutic potential.BMC Urol. 2024 Sep 12;24(1):198. doi: 10.1186/s12894-024-01585-7. BMC Urol. 2024. PMID: 39261818 Free PMC article. Review.
Cited by
-
Relationship between pyroptosis-mediated inflammation and the pathogenesis of prostate disease.Front Med (Lausanne). 2023 Jan 19;10:1084129. doi: 10.3389/fmed.2023.1084129. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36744134 Free PMC article. Review.
-
The Possibility and Molecular Mechanisms of Cell Pyroptosis After Cerebral Ischemia.Neurosci Bull. 2018 Dec;34(6):1131-1136. doi: 10.1007/s12264-018-0294-7. Epub 2018 Oct 10. Neurosci Bull. 2018. PMID: 30306532 Free PMC article. Review. No abstract available.
-
Peroxiredoxins as Potential Targets for Cardiovascular Disease.Antioxidants (Basel). 2021 Aug 3;10(8):1244. doi: 10.3390/antiox10081244. Antioxidants (Basel). 2021. PMID: 34439492 Free PMC article. Review.
-
Altered Cortical Palmitoylation Induces Widespread Molecular Disturbances in Parkinson's Disease.Int J Mol Sci. 2022 Nov 14;23(22):14018. doi: 10.3390/ijms232214018. Int J Mol Sci. 2022. PMID: 36430497 Free PMC article.
-
The roles of pyroptosis in genitourinary diseases.Int Urol Nephrol. 2024 May;56(5):1515-1523. doi: 10.1007/s11255-023-03894-6. Epub 2023 Dec 16. Int Urol Nephrol. 2024. PMID: 38103146 Free PMC article. Review.
References
-
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–79. - PubMed
-
- Emberton M, Fitzpatrick JM, Rees J. Risk stratification for benign prostatic hyperplasia (BPH) treatment. BJU Int. 2011;107:876–80. - PubMed
-
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–98. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

