Polysaccharides from Chrysanthemum morifolium Ramat ameliorate colitis rats by modulating the intestinal microbiota community
- PMID: 29113344
- PMCID: PMC5655239
- DOI: 10.18632/oncotarget.20477
Polysaccharides from Chrysanthemum morifolium Ramat ameliorate colitis rats by modulating the intestinal microbiota community
Abstract
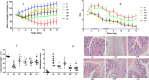
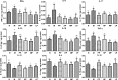
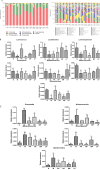
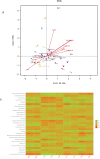
The gut microflora dysbiosis has been closely related with the inflammatory bowel disease (IBD). In this study, the effect of polysaccharides from Chrysanthemum morifolium Ramat on the gut microbiota was evaluated by ulcerative colitis (UC) rat model. Physiological and pathological analyses suggested that Chrysanthemum polysaccharides possessed notably protective effects on UC in vivo. Based on the Illumina MiSeq platform, 16S rRNA sequencing of the rat colonic contents indicated that the intestinal flora structure remarkably changed in the model rats and the tendency was alleviated to a certain degree by treatment with different dosages of Chrysanthemum polysaccharides. In normal groups, there were more Firmicutes than Bacteroidetes, but this change lost at the pathological state. Following Chrysanthemum polysaccharides, rising Firmicutes/Bacteroidetes ratio was validated. Besides the microbial diversity and the community richness of the UC rats were improved by Chrysanthemum polysaccharides, the composition of intestinal microflora in the model group were also restored after oral administration of Chrysanthemum polysaccharides. The abundance of opportunistic pathogens was decreased (Escherichia, Enterococcus and Prevotella), while the levels of protective bacteria such as Butyricicoccus and Clostridium (butyrate-producing bacteria), Lactobacillus and Bifidobacterium (probiotics), Lachnospiraceae and Rikenellaceae elevated in various degrees. Correlation analysis between intestinal flora and biochemical factors suggested that the relative abundance of protective bacteria was positively correlated with the levels of anti-inflammatory cytokines such as IL-4, IL-10 and IL-11, while aggressive bacteria were positively correlated with proinflammatory cytokine such as IL-23、IL-6、 IF-17、TNF-α、IL-1β and IFN-γ. The above results showed that the intestinal flora were closely related to the secretion and expression of cytokines in the body, and they interacted with each other to regulate immune function. Thus, Chrysanthemum polysaccharides could ameliorate ulcerative colitis by fostering beneficial intestinal flora growth, modulating the balance of intestinal microecology and restoring the immune system.
Keywords: 16S rRNA; chrysanthemum polysaccharides; microbial diversity; short chain fatty acids; ulcerative colitis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


Similar articles
-
Simultaneous determination of six short-chain fatty acids in colonic contents of colitis mice after oral administration of polysaccharides from Chrysanthemum morifolium Ramat by gas chromatography with flame ionization detector.J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Sep 1;1029-1030:88-94. doi: 10.1016/j.jchromb.2016.07.002. Epub 2016 Jul 4. J Chromatogr B Analyt Technol Biomed Life Sci. 2016. PMID: 27428450
-
Periplaneta americana Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Rats by Keap1/Nrf-2 Activation, Intestinal Barrier Function, and Gut Microbiota Regulation.Front Pharmacol. 2018 Aug 22;9:944. doi: 10.3389/fphar.2018.00944. eCollection 2018. Front Pharmacol. 2018. PMID: 30186174 Free PMC article.
-
Panax quinquefolius polysaccharides ameliorate ulcerative colitis in mice induced by dextran sulfate sodium.Front Immunol. 2023 Jun 21;14:1161625. doi: 10.3389/fimmu.2023.1161625. eCollection 2023. Front Immunol. 2023. PMID: 37415978 Free PMC article.
-
Traditional Chinese Medicine: A promising strategy to regulate the imbalance of bacterial flora, impaired intestinal barrier and immune function attributed to ulcerative colitis through intestinal microecology.J Ethnopharmacol. 2024 Jan 10;318(Pt A):116879. doi: 10.1016/j.jep.2023.116879. Epub 2023 Jul 5. J Ethnopharmacol. 2024. PMID: 37419224 Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Lentinan improves intestinal inflammation and gut dysbiosis in antibiotics-induced mice.Sci Rep. 2022 Nov 15;12(1):19609. doi: 10.1038/s41598-022-23469-2. Sci Rep. 2022. PMID: 36380080 Free PMC article.
-
Agrocybe cylindracea Polysaccharides Ameliorate DSS-Induced Colitis by Restoring Intestinal Barrier Function and Reprogramming Immune Homeostasis via the Gut-Liver Axis.Int J Mol Sci. 2025 Jul 16;26(14):6805. doi: 10.3390/ijms26146805. Int J Mol Sci. 2025. PMID: 40725052 Free PMC article.
-
The Potential Role of Plant Polysaccharides in Treatment of Ulcerative Colitis.Pharmaceutics. 2024 Aug 16;16(8):1073. doi: 10.3390/pharmaceutics16081073. Pharmaceutics. 2024. PMID: 39204418 Free PMC article. Review.
-
Traditional Chinese medicine to improve immune imbalance of asthma: focus on the adjustment of gut microbiota.Front Microbiol. 2024 Oct 1;15:1409128. doi: 10.3389/fmicb.2024.1409128. eCollection 2024. Front Microbiol. 2024. PMID: 39411430 Free PMC article. Review.
-
Treatment Effects of Natural Products on Inflammatory Bowel Disease In Vivo and Their Mechanisms: Based on Animal Experiments.Nutrients. 2023 Feb 18;15(4):1031. doi: 10.3390/nu15041031. Nutrients. 2023. PMID: 36839389 Free PMC article. Review.
References
-
- Pizzi LT, Weston CM, Goldfarb NI, Moretti D, Cobb N, Howell JB, Infantolino A, Dimarino AJ, Cohen S. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:47–52. - PubMed
-
- Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N, Brostoff J, Parkhill J, Dougan G, Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7–19. - PMC - PubMed
-
- Kucharzik T, Maaser C, Lugering A, Kagnoff M, Mayer L, Targan S, Domschke W. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1082. - PubMed
-
- Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med. 2008;263:597–606. - PubMed
-
- Mar JS, Nagalingam NA, Song YL, Onizawa M, Lee JW, Lynch SV. Amelioration of DSS-induced murine colitis by VSL#3 supplementation is primarily associated with changes in ileal microbiota composition. Gut Microbes. 2014;5:494–503. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

