PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer's disease mice
- PMID: 29113362
- PMCID: PMC5655257
- DOI: 10.18632/oncotarget.20944
PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer's disease mice
Abstract
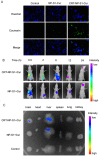
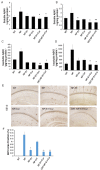
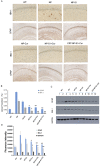
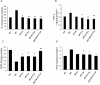
Alzheimer's disease (AD) is the most common form of dementia, characterized by the formation of extracellular senile plaques and neuronal loss caused by amyloid β (Aβ) aggregates in the brains of AD patients. Conventional strategies failed to treat AD in clinical trials, partly due to the poor solubility, low bioavailability and ineffectiveness of the tested drugs to cross the blood-brain barrier (BBB). Moreover, AD is a complex, multifactorial neurodegenerative disease; one-target strategies may be insufficient to prevent the processes of AD. Here, we designed novel kind of poly(lactide-co-glycolic acid) (PLGA) nanoparticles by loading with Aβ generation inhibitor S1 (PQVGHL peptide) and curcumin to target the detrimental factors in AD development and by conjugating with brain targeting peptide CRT (cyclic CRTIGPSVC peptide), an iron-mimic peptide that targets transferrin receptor (TfR), to improve BBB penetration. The average particle size of drug-loaded PLGA nanoparticles and CRT-conjugated PLGA nanoparticles were 128.6 nm and 139.8 nm, respectively. The results of Y-maze and new object recognition test demonstrated that our PLGA nanoparticles significantly improved the spatial memory and recognition in transgenic AD mice. Moreover, PLGA nanoparticles remarkably decreased the level of Aβ, reactive oxygen species (ROS), TNF-α and IL-6, and enhanced the activities of super oxide dismutase (SOD) and synapse numbers in the AD mouse brains. Compared with other PLGA nanoparticles, CRT peptide modified-PLGA nanoparticles co-delivering S1 and curcumin exhibited most beneficial effect on the treatment of AD mice, suggesting that conjugated CRT peptide, and encapsulated S1 and curcumin exerted their corresponding functions for the treatment.
Keywords: Alzheimer’s disease; curcumin; nanoparticles; peptide; β-amyloid.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declared that they have no conflicts of interest to this work.
Figures



Similar articles
-
A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer's disease.J Photochem Photobiol B. 2019 Jan;190:98-102. doi: 10.1016/j.jphotobiol.2018.11.008. Epub 2018 Nov 15. J Photochem Photobiol B. 2019. PMID: 30504054
-
Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates.Int J Pharm. 2017 Jun 30;526(1-2):413-424. doi: 10.1016/j.ijpharm.2017.05.015. Epub 2017 May 8. Int J Pharm. 2017. PMID: 28495580
-
Blood-Brain Barrier Penetrating Biologic TNF-α Inhibitor for Alzheimer's Disease.Mol Pharm. 2017 Jul 3;14(7):2340-2349. doi: 10.1021/acs.molpharmaceut.7b00200. Epub 2017 May 31. Mol Pharm. 2017. PMID: 28514851
-
Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer's disease.Br J Nutr. 2016 Feb 14;115(3):449-65. doi: 10.1017/S0007114515004687. Epub 2015 Dec 14. Br J Nutr. 2016. PMID: 26652155 Review.
-
Nanoliposomes as a Therapeutic Tool for Alzheimer's Disease.Front Synaptic Neurosci. 2020 May 25;12:20. doi: 10.3389/fnsyn.2020.00020. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 32523525 Free PMC article. Review.
Cited by
-
Significance of native PLGA nanoparticles in the treatment of Alzheimer's disease pathology.Bioact Mater. 2022 Jul 15;17:506-525. doi: 10.1016/j.bioactmat.2022.05.030. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 36330076 Free PMC article.
-
Potential Applications of Natural Components of Traditional Chinese Medicine Delivery via Nanoparticle Drug Delivery Systems in the Treatment of Alzheimer's Disease.Int J Nanomedicine. 2025 Jun 17;20:7781-7810. doi: 10.2147/IJN.S525960. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40546798 Free PMC article. Review.
-
Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges.Adv Sci (Weinh). 2021 Mar 7;8(10):2003937. doi: 10.1002/advs.202003937. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026447 Free PMC article. Review.
-
Advancements in the Application of Nanomedicine in Alzheimer's Disease: A Therapeutic Perspective.Int J Mol Sci. 2023 Sep 13;24(18):14044. doi: 10.3390/ijms241814044. Int J Mol Sci. 2023. PMID: 37762346 Free PMC article. Review.
-
Curcumin's Nanomedicine Formulations for Therapeutic Application in Neurological Diseases.J Clin Med. 2020 Feb 5;9(2):430. doi: 10.3390/jcm9020430. J Clin Med. 2020. PMID: 32033365 Free PMC article. Review.
References
-
- Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–63. https://doi.org/10.1038/nm1113 - DOI - PubMed
-
- Sala Frigerio C, De Strooper B. Alzheimer's disease mechanisms and emerging roads to novel therapeutics. Annu Rev Neurosci. 2016;39:57–79. https://doi.org/10.1146/annurev-neuro-070815-014015 - DOI - PubMed
-
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. https://doi.org/10.15252/emmm.201606210 - DOI - PMC - PubMed
-
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–84. https://doi.org/10.1111/j.1471-4159.2006.04426.x - DOI - PubMed
-
- Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B, Rockenstein E, Trejo M, Platoshyn O, Yuan JX, Masliah E. Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer's and Parkinson's diseases. PLoS One. 2008;3:e3135. https://doi.org/10.1371/journal.pone.0003135 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

