Change in risk of breast cancer after receiving hormone replacement therapy by considering effect-modifiers: a systematic review and dose-response meta-analysis of prospective studies
- PMID: 29113371
- PMCID: PMC5655266
- DOI: 10.18632/oncotarget.20154
Change in risk of breast cancer after receiving hormone replacement therapy by considering effect-modifiers: a systematic review and dose-response meta-analysis of prospective studies
Abstract
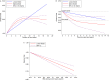
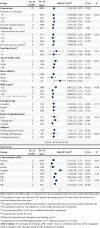
We synthesize the current literatures and use the power of meta-analysis to examine trends on association between hormone replacement therapy (HRT) and the risk of breast cancer (BC). We performed a comprehensive literature search using PubMed, EMBASE, and Web of Science from their inception until Jan 2017. Prospective studies that provided adjusted risk estimates of HRT and BC risk were eligible. Categorical and dose-response meta-analyses followed the PRISMA were conducted using random effects model and restricted cubic spline model, respectively. Forty-seven publications from thirty-five unique studies were included, involving 3,898,376 of participants and 87,845 of BC cases. Compared with non-users, RR for current estrogen-only therapy (ET) users was 1.14 (95% confidence interval (CI) = 1.05-1.22), and for per year increases was 1.02 (95% CI = 1.02-1.02). Moreover, RR for current estrogen plus progestin therapy (EPT) users was 1.76, (95% CI = 1.56-1.96), and for per year increases was 1.08 (95% CI = 1.08-1.08). Dose-response analyses revealed 8-10 years' onset peaks, and indicated residual increased BC risk remained after stopping use of ET regimen rather than for EPT. Effect-modifiers like BMI, duration of use, race/ethnicity, routes of administration were recognized. In Conclusions, current use of EP or EPT and ever use of tibolone are associated with an elevated risk of BC. Compared with slim HRT users and non-users, lower BC risks were found among overweight/obese HRT users and former EPT users, respectively. Both ET and EPT users are associated with higher risk of lobular BC than ductal BC, and more ER-positive than negative BC cases were detected among EPT users.
Keywords: body mass index; breast cancer; estrogen plus progestin therapy; estrogen-alone therapy; hormone replacement therapy.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that there are no conflicts of interest.
Figures



References
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. - PubMed
-
- Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Galichet L, Cogliano V. A review of human carcinogens--Part A: pharmaceuticals. Lancet Oncol. 2009;10:13–4. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. https://doi.org/10.3322/caac.21332 - DOI - PubMed
-
- Roman M, Sakshaug S, Graff-Iversen S, Vangen S, Weiderpass E, Ursin G, Hofvind S. Postmenopausal hormone therapy and the risk of breast cancer in Norway. Int J Cancer. 2016;138:584–93. https://doi.org/10.1002/ijc.29810 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

