Tacrolimus dose requirement based on the CYP3A5 genotype in renal transplant patients
- PMID: 29113387
- PMCID: PMC5655282
- DOI: 10.18632/oncotarget.18150
Tacrolimus dose requirement based on the CYP3A5 genotype in renal transplant patients
Erratum in
-
Correction: Tacrolimus dose requirement based on the CYP3A5 genotype in renal transplant patients.Oncotarget. 2020 Apr 21;11(16):1474-1477. doi: 10.18632/oncotarget.27561. eCollection 2020 Apr 21. Oncotarget. 2020. PMID: 32363004 Free PMC article.
Abstract
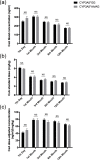
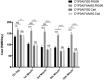
Tacrolimus (FK506) and cyclosporine A (CsA) are widely used to protect graft function after renal transplantation. The aim of the present study is to determine whether the single nucleotide polymorphism of CYP3A5 is a predictive index of FK506 dose requirement, and also the selection yardstick of FK506 or CsA treatment.We tested archival peripheral blood of 218 kidney recipients for CYP3A5 genotyping with PCR-SSP. Meanwhile, the dose of FK506 and CsA was recorded, blood concentration of the drugs was measured, and graft outcome was monitored.These results indicate that CYP3A5*AA/AG carriers need higher FK506 dose than CYP3A5*GG homozygote to achieve the target blood concentration. For CYP3A5*GG carriers, taking FK506 or CsA are both advisable. CYP3A5*AA/AG carriers preferred to CsA treatment depending on the graft outcomes and drug costs. CYP3A5 genotyping is a new approach to detecting FK506 dose requirement and a predictive index for the FK506 or CsA treatment selection in kidney recipients.
Keywords: CYP3A5; FK506; acute rejection; renal transplantation.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no competing financial interests.
Figures


Similar articles
-
Effect of CYP3A5 polymorphism on the pharmacokinetics of tacrolimus and acute rejection in renal transplant recipients: experience at a single centre.Int J Clin Pract Suppl. 2015 May;(183):16-22. doi: 10.1111/ijcp.12662. Int J Clin Pract Suppl. 2015. PMID: 26177012
-
Comparison of tacrolimus and cyclosporin A in CYP3A5 expressing Chinese de novo kidney transplant recipients: a 2-year prospective study.Int J Clin Pract Suppl. 2015 May;(183):43-52. doi: 10.1111/ijcp.12666. Int J Clin Pract Suppl. 2015. PMID: 26177348 Clinical Trial.
-
Impact of POR*28 on the pharmacokinetics of tacrolimus and cyclosporine A in renal transplant patients.Ther Drug Monit. 2014 Feb;36(1):71-9. doi: 10.1097/FTD.0b013e31829da6dd. Ther Drug Monit. 2014. PMID: 24061445 Clinical Trial.
-
Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II.Clin Pharmacokinet. 2010 Apr;49(4):207-21. doi: 10.2165/11317550-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20214406 Review.
-
Effects of genetic polymorphisms on the pharmacokinetics of calcineurin inhibitors.Am J Health Syst Pharm. 2006 Dec 1;63(23):2340-8. doi: 10.2146/ajhp060080. Am J Health Syst Pharm. 2006. PMID: 17106006 Review.
Cited by
-
Relationship between TGF-β1 + 869 T/C and + 915 G/C gene polymorphism and risk of acute rejection in renal transplantation recipients.BMC Med Genet. 2019 Jun 25;20(1):113. doi: 10.1186/s12881-019-0847-2. BMC Med Genet. 2019. PMID: 31238890 Free PMC article. Review.
-
CYP3A5 and CYP3A7 genetic polymorphisms affect tacrolimus concentration in pediatric patients with nephrotic range proteinuria.Eur J Clin Pharmacol. 2019 Nov;75(11):1533-1540. doi: 10.1007/s00228-019-02726-w. Epub 2019 Aug 10. Eur J Clin Pharmacol. 2019. PMID: 31401678
-
CYP3A5 Genotype-Dependent Drug-Drug Interaction Between Tacrolimus and Nifedipine in Chinese Renal Transplant Patients.Front Pharmacol. 2021 Jul 5;12:692922. doi: 10.3389/fphar.2021.692922. eCollection 2021. Front Pharmacol. 2021. PMID: 34290611 Free PMC article.
-
Microfluidics as a Novel Tool for Biological and Toxicological Assays in Drug Discovery Processes: Focus on Microchip Electrophoresis.Micromachines (Basel). 2020 Jun 15;11(6):593. doi: 10.3390/mi11060593. Micromachines (Basel). 2020. PMID: 32549277 Free PMC article. Review.
References
-
- MacPhee IA, Holt DW. A pharmacogenetic strategy for immunosuppression based on the CYP3A5 genotype. Transplantation. 2008;85:163–5. - PubMed
-
- Vadivel N, Garg A, Holt DW, Chang RW, MacPhee IA. Tacrolimus dose in black renal transplant recipients. Transplantation. 2007;83:997–999. - PubMed
-
- Macphee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, Goldberg L, Holt DW. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002;74:1486–1489. - PubMed
-
- Song J, Kim MG, Choi B, Han NY, Yun HY, Yoon JH, Oh JM. CYP3A5 polymorphism effect on cyclosporine pharmacokinetics in living donor renal transplant recipients: analysis by population pharmacokinetics. Ann Pharmacother. 2012;46:1141–1151. - PubMed
-
- Peng W, Chen J, Jiang Y, Wu J, Shou Z, He Q, Wang Y, Chen Y, Wang H. Urinary fractalkine is a marker of acute rejection. Kidney Int. 2008;74:1454–1460. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

