Adipose-Derived Mesenchymal Stem Cells from the Elderly Exhibit Decreased Migration and Differentiation Abilities with Senescent Properties
- PMID: 29113467
- PMCID: PMC5680952
- DOI: 10.1177/0963689717721221
Adipose-Derived Mesenchymal Stem Cells from the Elderly Exhibit Decreased Migration and Differentiation Abilities with Senescent Properties
Abstract
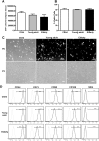
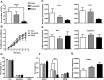
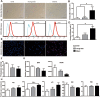
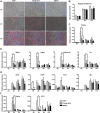
Adipose-derived stem cells (ASCs) can be applied extensively in the clinic because they can be easily isolated and cause less donor-site morbidity; however, their application can be complicated by patient-specific factors, such as age and harvest site. In this study, we systematically evaluated the effects of age on the quantity and quality of human adipose-derived mesenchymal stem cells (hASCs) isolated from excised chest subcutaneous adipose tissue and investigated the underlying molecular mechanism. hASCs were isolated from donors of 3 different age-groups (i.e., child, young adult, and elderly). hASCs are available from individuals across all age-groups and maintain mesenchymal stem cell (MSC) characteristics. However, the increased age of the donors was found to have a significant negative effect on hASCs frequency base on colony-forming unit fibroblasts assay. Moreover, there is a decline in both stromal vascular fraction (SVF) cell yield and the proliferation rate of hASCs with increasing age, although this relationship is not significant. Aging increases cellular senescence, which is manifested as an increase in SA-β-gal-positive cells, increased mitochondrial-specific reactive oxygen species (ROS) production, and the expression of p21 in the elderly. Further, advancing age was found to have a significant negative effect on the adipogenic and osteogenic differentiation potentials of hASCs, particularly at the early and mid-stages of induction, suggesting a slower response to the inducing factors of hASCs from elderly donors. Finally, impaired migration ability was also observed in the elderly group and was determined to be associated with decreased expression of chemokine receptors, such as CXCR4 and CXCR7. Taken together, these results suggest that, while hASCs from different age populations are phenotypically similar, they present major differences at the functional level. When considering potential applications of hASCs in cell-based therapeutic strategies, the negative influence of age on hASC differentiation potential and migration abilities should be taken seriously.
Keywords: aging; cell senescence; differentiation; human adipose-derived mesenchymal stem cells (hASCs); migration.
Conflict of interest statement
Figures


References
-
- Gimble JM, Bunnell BA, Floyd ZE. Prospecting for adipose progenitor cell biomarkers: biopanning for gold with in vivo phage display. Cell Stem Cell. 2011;9(1):1–2. - PubMed
-
- Nicpon J, Marycz K, Grzesiak J. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol J Vet Sci. 2013;16(4):753–754. - PubMed
-
- Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A, Zissimopoulos A, Boussios N, Dardzinski B, Gritzalis D, et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med. 2013;11:171. - PMC - PubMed
-
- Murohara T, Shintani S, Kondo K. Autologous adipose-derived regenerative cells for therapeutic angiogenesis. Curr Pharm Des. 2009;15(24):2784–2790. - PubMed
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

