Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial
- PMID: 29113968
- PMCID: PMC5672899
- DOI: 10.1136/bmj.j4784
Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial
Erratum in
-
Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial.BMJ. 2017 Nov 13;359:j5268. doi: 10.1136/bmj.j5268. BMJ. 2017. PMID: 29133285 Free PMC article. No abstract available.
Abstract
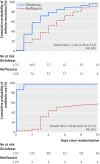
Objective To investigate whether symptomatic treatment with non-steroidal anti-inflammatory drugs (NSAIDs) is non-inferior to antibiotics in the treatment of uncomplicated lower urinary tract infection (UTI) in women, thus offering an opportunity to reduce antibiotic use in ambulatory care.Design Randomised, double blind, non-inferiority trial.Setting 17 general practices in Switzerland.Participants 253 women with uncomplicated lower UTI were randomly assigned 1:1 to symptomatic treatment with the NSAID diclofenac (n=133) or antibiotic treatment with norfloxacin (n=120). The randomisation sequence was computer generated, stratified by practice, blocked, and concealed using sealed, sequentially numbered drug containers.Main outcome measures The primary outcome was resolution of symptoms at day 3 (72 hours after randomisation and 12 hours after intake of the last study drug). The prespecified principal secondary outcome was the use of any antibiotic (including norfloxacin and fosfomycin as trial drugs) up to day 30. Analysis was by intention to treat.Results 72/133 (54%) women assigned to diclofenac and 96/120 (80%) assigned to norfloxacin experienced symptom resolution at day 3 (risk difference 27%, 95% confidence interval 15% to 38%, P=0.98 for non-inferiority, P<0.001 for superiority). The median time until resolution of symptoms was four days in the diclofenac group and two days in the norfloxacin group. A total of 82 (62%) women in the diclofenac group and 118 (98%) in the norfloxacin group used antibiotics up to day 30 (risk difference 37%, 28% to 46%, P<0.001 for superiority). Six women in the diclofenac group (5%) but none in the norfloxacin group received a clinical diagnosis of pyelonephritis (P=0.03).Conclusion Diclofenac is inferior to norfloxacin for symptom relief of UTI and is likely to be associated with an increased risk of pyelonephritis, even though it reduces antibiotic use in women with uncomplicated lower UTI.Trial registration ClinicalTrials.gov NCT01039545.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

Comment in
-
Antibiotics or NSAIDs for uncomplicated urinary tract infection?BMJ. 2017 Nov 8;359:j5037. doi: 10.1136/bmj.j5037. BMJ. 2017. PMID: 29117972 No abstract available.
-
Urinary infections are complex and hard to treat.BMJ. 2017 Dec 18;359:j5766. doi: 10.1136/bmj.j5766. BMJ. 2017. PMID: 29254999 No abstract available.
-
Re: Symptomatic Treatment of Uncomplicated Lower Urinary Tract Infections in the Ambulatory Setting: Randomised, Double Blind Trial.J Urol. 2018 Apr;199(4):874-875. doi: 10.1016/j.juro.2018.01.018. Epub 2018 Jan 9. J Urol. 2018. PMID: 29642324 No abstract available.
References
-
- Organization WH. Global Action Plan on Antimicrobial Resistance.WHO Library Cataloguing-in-Publication Data, 2015.
-
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002;113(Suppl 1A):5-13. 10.1016/S0002-9343(02)01054-9 pmid:12113866. - DOI - PubMed
-
- Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am 2003;17:227-41. 10.1016/S0891-5520(03)00005-9 pmid:12848468. - DOI - PubMed
-
- Mühlemann K. Surveillance of antibiotic prescription in the outpatient setting using the national Sentinel network (OP-159, http://www.smw.ch/docs/PdfContent/smw-12931.pdf ). WONCA World Organization of National Colleges, Academies and Academic Associations of General Practitioners/Family Physicians; 2009; Basel. Switzerland; 2009.
-
- Lundborg CS, Olsson E, Mölstad S. Swedish Study Group on Antibiotic Use. Antibiotic prescribing in outpatients: a 1-week diagnosis-prescribing study in 5 counties in Sweden. Scand J Infect Dis 2002;34:442-8. 10.1080/00365540110080647 pmid:12160172. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
