An ultra-stable single-chain insulin analog resists thermal inactivation and exhibits biological signaling duration equivalent to the native protein
- PMID: 29114035
- PMCID: PMC5766902
- DOI: 10.1074/jbc.M117.808626
An ultra-stable single-chain insulin analog resists thermal inactivation and exhibits biological signaling duration equivalent to the native protein
Abstract
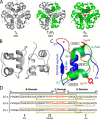
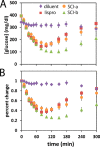
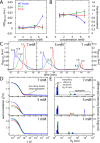
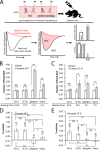
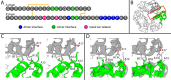
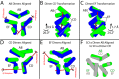
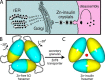
Thermal degradation of insulin complicates its delivery and use. Previous efforts to engineer ultra-stable analogs were confounded by prolonged cellular signaling in vivo, of unclear safety and complicating mealtime therapy. We therefore sought an ultra-stable analog whose potency and duration of action on intravenous bolus injection in diabetic rats are indistinguishable from wild-type (WT) insulin. Here, we describe the structure, function, and stability of such an analog, a 57-residue single-chain insulin (SCI) with multiple acidic substitutions. Cell-based studies revealed native-like signaling properties with negligible mitogenic activity. Its crystal structure, determined as a novel zinc-free hexamer at 2.8 Å, revealed a native insulin fold with incomplete or absent electron density in the C domain; complementary NMR studies are described in the accompanying article. The stability of the analog (ΔGU 5.0(±0.1) kcal/mol at 25 °C) was greater than that of WT insulin (3.3(±0.1) kcal/mol). On gentle agitation, the SCI retained full activity for >140 days at 45 °C and >48 h at 75 °C. These findings indicate that marked resistance to thermal inactivation in vitro is compatible with native duration of activity in vivo Further, whereas WT insulin forms large and heterogeneous aggregates above the standard 0.6 mm pharmaceutical strength, perturbing the pharmacokinetic properties of concentrated formulations, dynamic light scattering, and size-exclusion chromatography revealed only limited SCI self-assembly and aggregation in the concentration range 1-7 mm Such a combination of favorable biophysical and biological properties suggests that SCIs could provide a global therapeutic platform without a cold chain.
Keywords: diabetes; hormone; protein engineering; protein structure; receptor tyrosine kinase.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
M.A.W. has equity in Thermalin Diabetes, LLC (Cleveland, OH), where he serves as Chief Innovation Officer; he has also been a consultant to Merck Research Laboratories and DEKA Research & Development Corp. N. B. P. and F. I.-B. are consultants to Thermalin Diabetes, LLC. Part of M.C.L.'s research is funded by Sanofi (Germany)
Figures
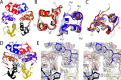


References
-
- Baker E. N., Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. M., Hubbard R. E., Isaacs N. W., and Reynolds C. D. (1988) The structure of 2Zn pig insulin crystals at 1.5 Å resolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 319, 369–456 - PubMed
-
- Rinderknecht E., and Humbel R. E. (1978) The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J. Biol. Chem. 253, 2769–2776 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

