Regulation and dysregulation of axon infrastructure by myelinating glia
- PMID: 29114067
- PMCID: PMC5716274
- DOI: 10.1083/jcb.201702150
Regulation and dysregulation of axon infrastructure by myelinating glia
Abstract
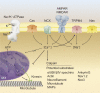
Axon loss and neurodegeneration constitute clinically debilitating sequelae in demyelinating diseases such as multiple sclerosis, but the underlying mechanisms of secondary degeneration are not well understood. Myelinating glia play a fundamental role in promoting the maturation of the axon cytoskeleton, regulating axon trafficking parameters, and imposing architectural rearrangements such as the nodes of Ranvier and their associated molecular domains. In the setting of demyelination, these changes may be reversed or persist as maladaptive features, leading to axon degeneration. In this review, we consider recent insights into axon-glial interactions during development and disease to propose that disruption of the cytoskeleton, nodal architecture, and other components of axon infrastructure is a potential mediator of pathophysiological damage after demyelination.
© 2017 Pan and Chan.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

