Epistatic interactions between mutations of TACI (TNFRSF13B) and TCF3 result in a severe primary immunodeficiency disorder and systemic lupus erythematosus
- PMID: 29114388
- PMCID: PMC5671988
- DOI: 10.1038/cti.2017.41
Epistatic interactions between mutations of TACI (TNFRSF13B) and TCF3 result in a severe primary immunodeficiency disorder and systemic lupus erythematosus
Abstract
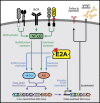
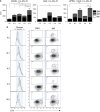
Common variable immunodeficiency disorders (CVID) are a group of primary immunodeficiencies where monogenetic causes account for only a fraction of cases. On this evidence, CVID is potentially polygenic and epistatic although there are, as yet, no examples to support this hypothesis. We have identified a non-consanguineous family, who carry the C104R (c.310T>C) mutation of the Transmembrane Activator Calcium-modulator and cyclophilin ligand Interactor (TACI, TNFRSF13B) gene. Variants in TNFRSF13B/TACI are identified in up to 10% of CVID patients, and are associated with, but not solely causative of CVID. The proband is heterozygous for the TNFRSF13B/TACI C104R mutation and meets the Ameratunga et al. diagnostic criteria for CVID and the American College of Rheumatology criteria for systemic lupus erythematosus (SLE). Her son has type 1 diabetes, arthritis, reduced IgG levels and IgA deficiency, but has not inherited the TNFRSF13B/TACI mutation. Her brother, homozygous for the TNFRSF13B/TACI mutation, is in good health despite profound hypogammaglobulinemia and mild cytopenias. We hypothesised that a second unidentified mutation contributed to the symptomatic phenotype of the proband and her son. Whole-exome sequencing of the family revealed a de novo nonsense mutation (T168fsX191) in the Transcription Factor 3 (TCF3) gene encoding the E2A transcription factors, present only in the proband and her son. We demonstrate mutations of TNFRSF13B/TACI impair immunoglobulin isotype switching and antibody production predominantly via T-cell-independent signalling, while mutations of TCF3 impair both T-cell-dependent and -independent pathways of B-cell activation and differentiation. We conclude that epistatic interactions between mutations of the TNFRSF13B/TACI and TCF3 signalling networks lead to the severe CVID-like disorder and SLE in the proband.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Abbott JK, Gelfand EW. Common variable immunodeficiency: diagnosis, management, and treatment. Immunol Allergy Clin North Am 2015; 35: 637–658. - PubMed
-
- Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol 2003; 4: 261–268. - PubMed
-
- van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med 2006; 354: 1901–1912. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
