Concordance Between Different Amyloid Immunoassays and Visual Amyloid Positron Emission Tomographic Assessment
- PMID: 29114726
- PMCID: PMC5822196
- DOI: 10.1001/jamaneurol.2017.2814
Concordance Between Different Amyloid Immunoassays and Visual Amyloid Positron Emission Tomographic Assessment
Abstract
Importance: Visual assessment of amyloid positron emission tomographic (PET) images has been approved by regulatory authorities for clinical use. Several immunoassays have been developed to measure β-amyloid (Aβ) 42 in cerebrospinal fluid (CSF). The agreement between CSF Aβ42 measures from different immunoassays and visual PET readings may influence the use of CSF biomarkers and/or amyloid PET assessment in clinical practice and trials.
Objective: To determine the concordance between CSF Aβ42 levels measured using 5 different immunoassays and visual amyloid PET analysis.
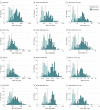
Design, setting, and participants: The study included 262 patients with mild cognitive impairment or subjective cognitive decline from the Swedish BioFINDER (Biomarkers for Identifying Neurodegenerative Disorders Early and Reliably) cohort (recruited from September 1, 2010, through December 31, 2014) who had undergone flutemetamol F 18 ([18F]flutemetamol)-labeled PET. Levels of CSF Aβ42 were analyzed using the classic INNOTEST and the newer modified INNOTEST, fully automated Lumipulse (FL), EUROIMMUN (EI), and Meso Scale Discovery (MSD) assays. Concentrations of CSF Aβ were assessed using an antibody-independent mass spectrometry-based reference measurement procedure.
Main outcomes and measures: The concordance of CSF Aβ42 levels and Aβ42:Aβ40 and Aβ42:tau ratios with visual [18F]flutemetamol PET status.
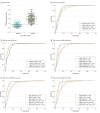
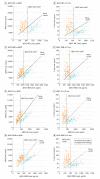
Results: Of 262 participants (mean [SD] age, 70.9 [5.5] years), 108 were women (41.2%) and 154 were men (58.8%). The mass spectrometry-derived Aβ42 values showed higher correlations with the modified Aβ42-INNOTEST (r = 0.97), Aβ42-FL (r = 0.93), Aβ42-EI (r = 0.93), and Aβ42-MSD (r = 0.95) assays compared with the classic Aβ42-INNOTEST assay (r = 0.88; P ≤ .01). The signal in the classic Aβ42-INNOTEST assay was partly quenched by recombinant Aβ1-40 peptide. However, the classic Aβ42-INNOTEST assay showed better concordance with visual [18F]flutemetamol PET status (area under the receiver operating characteristic curve [AUC], 0.92) compared with the newer assays (AUCs, 0.87-0.89; P ≤ .01). The accuracies of the newer assays improved significantly when Aβ42:Aβ40 (AUCs, 0.93-0.95; P ≤ .01), Aβ42 to total tau (T-tau) (AUCs, 0.94; P ≤ .05), or Aβ42 to phosphorylated tau (P-tau) (AUCs, 0.94-0.95; P ≤ .001) ratios were used. A combination of the Aβ42:Aβ40 ratio and T-tau or P-tau level did not improve the accuracy compared with the ratio alone.
Conclusions and relevance: Concentrations of CSF Aβ42 derived from the new immunoassays (modified INNOTEST, FL, EI, and MSD) may correlate better with the antibody-independent mass spectrometry-based reference measurement procedure and may show improved agreement with visual [18F]flutemetamol PET assessment when using the Aβ42:Aβ40 or Aβ42:tau ratios. These findings suggest the benefit of implementing the CSF Aβ42:Aβ40 or Aβ42:tau ratios as a biomarker of amyloid deposition in clinical practice and trials.
Conflict of interest statement
Figures
References
-
- Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36(5):297-309. - PubMed
-
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60(4):652-656. - PubMed
-
- Tapiola T, Alafuzoff I, Herukka SK, et al. . Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382-389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

